by Whitney Zi Yee Woo, Singha Suborna, Tabia Tarannum, Jonathan Machado, Kevin Pena, Fatoumata Kone, Sean Fernandez, Crystal Matos, Kayum Muhammed and Dr. John Nobleman
Steam distillation was employed to extract limonene, the major volatile component of orange peels. The extract was purified using a 50:50 mixture of hexane and t-butyl methyl ether. Purity was assessed via Thin Layer Chromatography (TLC), while IR spectroscopy confirmed functional groups. The high Rf value indicated successful extraction with minimal impurities. This study demonstrates the efficiency of steam distillation for isolating essential oils and emphasizes the importance of solvent systems in chemical purity analysis, along with a discussion of limonene's acid-base mechanisms. The Gaussian methodology, integrating quantum mechanical calculations with NMR prediction techniques, was employed to accurately estimate the NMR spectra of limonene and its reaction products.
Keywords:Steam distillation, limonene, TLC, purification, hexane, t-butyl methyl ether, toluene, ethanol, IR spectrum, chemical reactions, non-conjugated diene, Gaussian, calculation, 1H and 13C NMR, structure prediction.
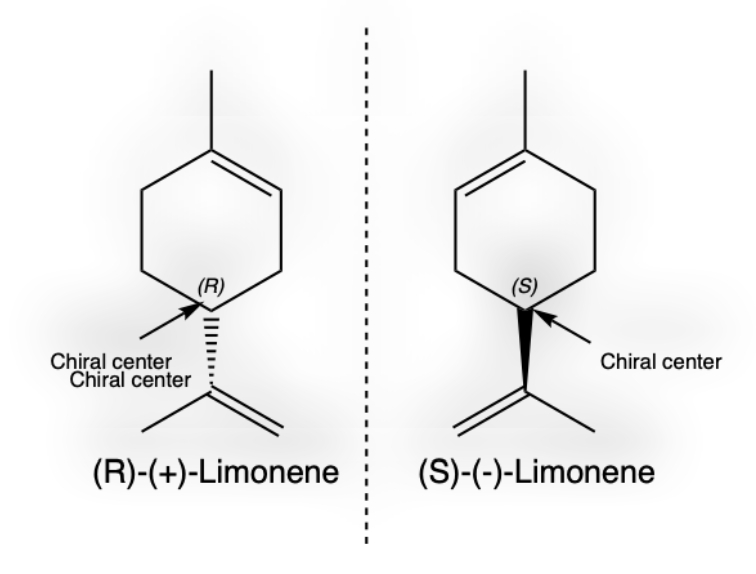 Figure 1: The chiral molecules of (R)- and (S)-Limonene enantiomers.
Figure 1: The chiral molecules of (R)- and (S)-Limonene enantiomers.
The orange peel contains a variety of substances, with one of the most notable being limonene (see Fig. 1), a monoterpene responsible for its characteristic citrus aroma. Limonene is a hydrophobic, nonpolar molecule found in the oil glands of orange peels and is commonly extracted through steam distillation [1,2]. The steam extraction, gives an oil that typically contains more than 90% of limonene. In addition to limonene, orange peels contain flavonoids such as hesperidin, which act as antioxidants; citric acid, which contributes a slight acidic nature; pectin, a polysaccharide often used as a gelling agent; essential oils, including other terpenes and aldehydes like citral and linalool; and carotenoids, pigments responsible for the orange color [3,4,5]. Limonene and (S)-(+)-Carvone, along with, is present in caraway and dill seed oils, contributing significantly to their distinctive aromas. Dill seed, derived from the dill plant (Anethum graveolens), is widely used as a spice for its slightly bitter flavor reminiscent of caraway and anise. Our ability to distinguish between enantiomers is due to the chiral nature of the odor receptors in our noses, which are linked to the nervous system. Figure 1 shows (+)-D-Limonene, a chiral molecule, and the orange tree's ability to produce the (R)-enantiomer [6]. Limonene (C₁₀H₁₆) comprises a 6-membered ring and two C=C double bonds, which act as nucleophilic centers in chemical reactions. It contains one chiral center (labeled as R or S); the four substituents around the chiral center can be arranged differently to yield either (+)- or (−)-limonene, each possessing distinct smells. The (+)-limonene form found in orange peel is known for its sweet, fresh citrus scent and is widely used in the food, fragrance, cosmetic industries, and its insecticidal properties contribute to its use in pest control [7]. Limonene is a stable terpene with a boiling point of 175–176°C and a melting point of –74.3°C [8]. It is slightly soluble in water around 13.8 mg/mL at 25°C but well soluble in the aprotic solvent such as acetone, dimethyl sulfoxide (DMSO).
Steam distillation is a specialized technique used to separate and purify organic compounds immiscible with water. Unlike traditional distillation, which follows Raoult's Law assuming miscibility, steam distillation works with immiscible liquids. Raoult's Law no longer applies, and the liquids establish independent vapor pressures. As a result, the total vapor pressure is the sum of the individual pressures, which leads to a consistent ratio of components in the vapor phase. This behavior makes traditional distillation ineffective for immiscible liquids but ideal for steam distillation.
The objective of this work is to extract limonene, the main component of orange oil, from orange peels using steam distillation. Limonene, a volatile and hydrophobic compound, is separated due to its immiscibility with water, preserving its chemical integrity at lower temperatures. The extraction is enhanced with the aid of a 50:50 hexane:t-butyl methyl ether mixture, followed by purification and analysis using TLC, IR and NMR spectroscopy to confirm the chemical structure of limonene.
The following materials were used in the experiment: fresh orange peels, distilled water, and a distillation apparatus. A separatory funnel, a 50:50 mixture of hexane and t-butyl methyl ether, and a 30:1 mixture of toluene and ethanol were also required. Boiling chips were used in the distillation process, and a sand bath was employed to control the temperature. Silica gel-coated TLC plates, a ring stand, and filter paper were used for thin-layer chromatography. A UV light source was used for visualization of the TLC spots. Other materials included beakers, aluminum foil, capillary tubes, a syringe needle, and a centrifuge tube.
To isolate limonene, a steam distillation apparatus was set up in the laboratory [9]. Orange peels were placed in a micro round-bottom flask, filling it halfway, along with a boiling chip. All glassware connections were secured, and a thermometer was attached. Distilled water was added portion by portion using a syringe needle during the distillation process to prevent the heated flask from drying. The distillate was directed into a receiver flask placed in an ice bath. The flask was heated gently until the mixture boiled, with the temperature monitored using the thermometer. The heat was adjusted to maintain a steady distillation rate while avoiding excessive foaming. Distilled water was periodically added to sustain steam generation until the receiver flask was half-filled. The heat was then turned off, and the apparatus was allowed to cool to room temperature.
Extraction of lemonene from Orange Peels: The extracted essential oil was transferred into a centrifuge tube, and an equal volume of a 50:50 hexane and t-butyl methyl ether mixture was added portion by portion. The mixture was gently shaken and allowed to separate into polar and non-polar layers. The lower aqueous layer was removed using a syringe, and calcium chloride (CaCl₂) was added to the organic layer to remove water molecules. After drying the solution, the solvents were removed by reducing the air pressure. The dried limonene was then used for the thin-layer chromatography (TLC) experiment, as shown in Fig. 2. The spots were visualized under UV light to confirm the absence of impurities and to compare the results with those obtained from the TLC analysis of the crude sample.

Figure 2: From left to right: Microscale steam distillation apparatus showing the product obtained, which appears yellowish in color due to slightly impurities come from carotenoids pigments responsible for the orange color; TLC plates displaying results under UV light using two different solvent systems. The TLC plate on the left employs a hexane/t-butyl methyl ether mixture (50:50), while the plate on the right uses a toluene/ethanol mixture (30:1). On the TLC plates: (-A) indicates the origin sample, representing residuals in flask. (-B) represents the product obtained after steam distillation and extraction. (-C) serves as the control substance, a pure polar compound adhering to the silica gel on the TLC plate.
Figure 2 shows the process of microscale steam destillation apparatus during the steam distillation and the clean-and-dry light-yelowish product was obtained after drying over CaCl2 to remove water and then liquid-liquid extraction process using a hexane/t-butyl methyl ether mixture (50:50); the combined solvent extracts was evaporated, usually under reduced pressure, finally the TLC plate developed using the mixture organic solvent systems; a toluene/ethanol mixture (30:1), while the plate on the right uses a hexane/t-butyl methyl ether mixture (50:50). Table 1 shows the TLC analysis (based on Fig. 2), where the aqueous layer (A), a mixture of substances, indicates the presence of limonene from the orange peel after steam distillation. Compound B (organic layer) shows that limonene, being non-polar, was successfully extracted and moved further along the TLC plate in the organic phase, with an Rf value of 0.48.
| Compound | Distance Traveled by Compound (cm) | Solvent front (cm) | Rf Value |
|---|---|---|---|
| After Sream destillation mix substances (A) | 3.6 | 7.5 | 0.48 |
| Limonene (B) | 5.6 | 7.5 | 0.75 |
Following the distillation, the organic layer containing limonene was extracted using a 50:50 mixture of hexane and t-butyl methyl ether. This solvent mixture was chosen because it is non-polar enough to dissolve limonene, yet sufficiently polar to separate any residual polar impurities. The organic extract was then dried, and the solvent was evaporated, leaving behind the purified limonene. The TLC was performed to analyze the purity of the limonene extract and confirm the success of the extraction process. Our experiment shows that the toluene/ethanol mixture in a 30:1 ratio much better solvent system where toluene is a non-polar solvent, which is ideal for dissolving non-polar compounds such as limonene, and ethanol, being slightly polar, can help resolve any residual polar impurities. This combination offers a better polarity gradient, providing a more distinct separation of limonene from other compounds as shown in Fig. 2 for comparison.
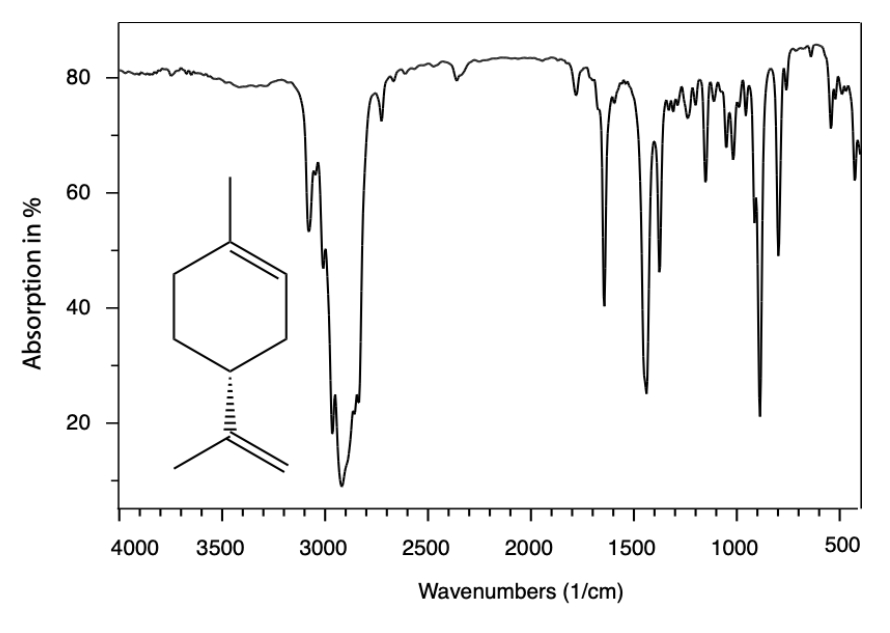
Figure 3: IR spectrum of Limonene. The IR spectroscopy typically cannot distinguish between enantiomers. This is because enantiomers have identical molecular structures, including bond vibrations, and thus show similar IR spectra. The calculated of dipole moment of limonene is about 0.555 Debay.
Figure 3 shows the IR vibration of limonene, where the IR spectroscopy typically cannot distinguish between enantiomers. This is because enantiomers have identical molecular structures, including bond vibrations, and thus show similar IR spectra. The only difference lies in their ability to rotate plane-polarized light, which IR spectroscopy does not detect. To distinguish between enantiomers, techniques like circular dichroism (CD) or optical rotation measurements are more effective.
The infrared (IR) spectrum of limonene, shown in Figure 3, provides a fingerprint of its molecular structure, characterized by functional groups and vibrational modes. Key features of the IR spectrum of limonene include the following: C-H Stretching (Alkene and Alkane): 3100–3000 cm⁻¹: Indicates =C-H stretching from the alkene group. 2950–2850 cm⁻¹: Represents C-H stretching from sp³-hybridized carbon atoms in the alkane portion. C=C Stretching (Alkene):1650–1600 cm⁻¹: Indicates the presence of the carbon-carbon double bond (C=C) from sp²-hybridized carbon atoms. C-H Bending (Alkane and Alkene): 1450 cm⁻¹: Represents CH₂ and CH₃ bending vibrations. 1375 cm⁻¹: Corresponds to CH₃ symmetric bending. C-H Out-of-Plane Bending (Alkene): 900–650 cm⁻¹: Indicates bending characteristic of the =C-H group in alkenes. Fingerprint Region: 1500–400 cm⁻¹: Displays a complex pattern of bending and stretching vibrations unique to limonene, making it useful for compound identification.
Limonene's IR spectrum reflects its monoterpene structure, which contains an isoprene unit and a terminal alkene group. The absence of significant peaks above 3200 cm⁻¹ confirms that it lacks hydroxyl (–OH) or amine (–NH) groups.
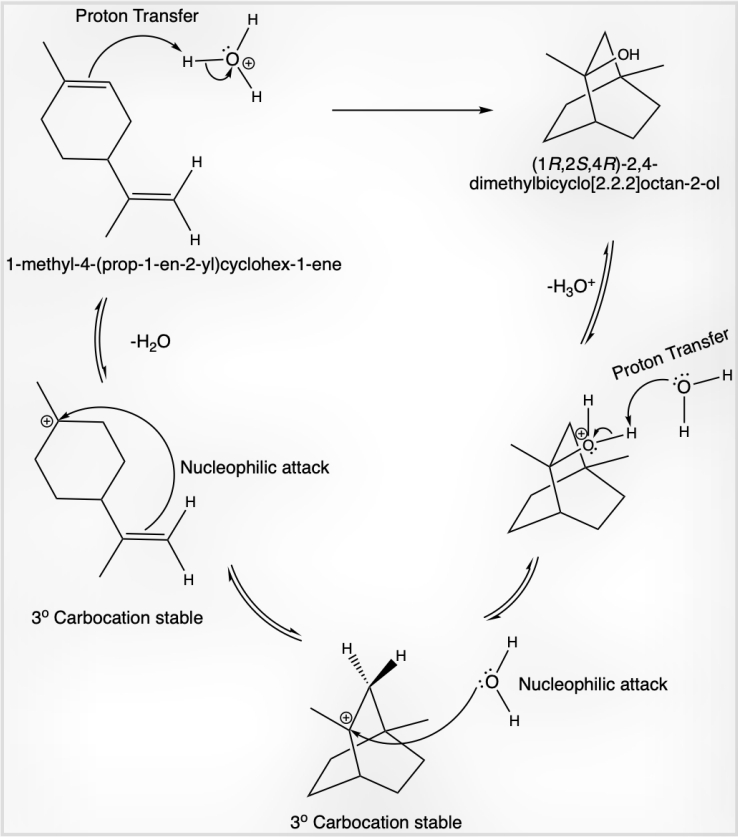
Figure 4: H₃O⁺ donates a proton, acting as an acid, while the alkene double bond functions as a base and accepts the proton. This generates a stable tertiary carbocation. The double bond of the alkene, acting as an internal nucleophile, attacks the tertiary carbocation, leading to the formation of a 2,4-dimethylbicyclo[2.2.2]octan-2-ylium intermediate. Water molecules then act as nucleophiles, attacking the carbocation to form a conjugate acid, which subsequently undergoes deprotonation to produce the final stable product.
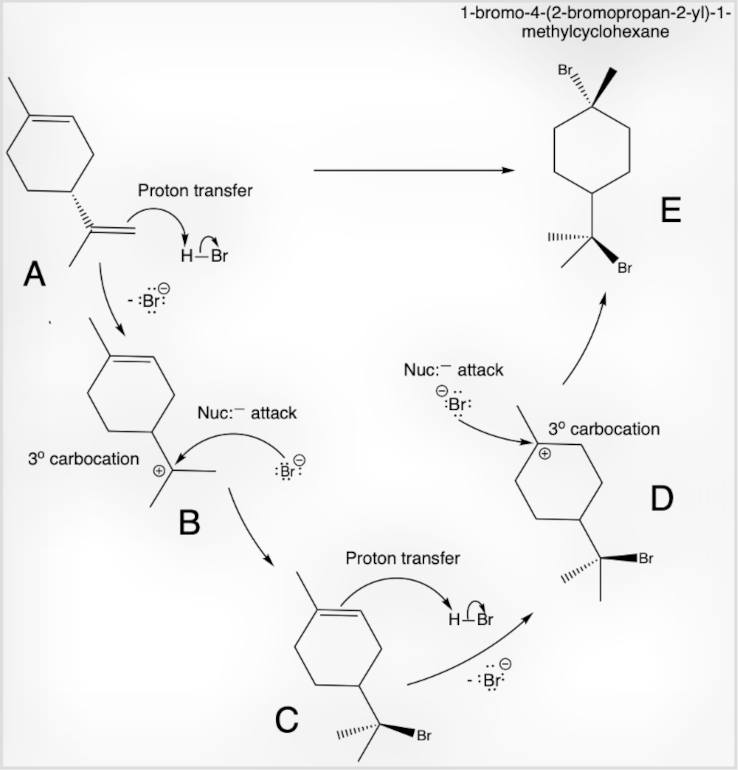
Figure 5: The less-substituted alkene double bond (A) acts as a base by accepting a proton in this proton-transfer reaction, with HBr serving as the acid (proton donor). A stable tertiary carbocation intermediate (B) forms following Markovnikov’s rule, which is then attacked by the nucleophilic bromide ion (Br⁻). The more-substituted double bond alkene (C) also acts as a base, accepting a proton from excess HBr, forming another tertiary carbocation intermediate (D). The bromide ion (Br⁻) readily attacks this carbocation, resulting in the formation of 1-bromo-4-(2-bromopropan-2-yl)-1-methylcyclohexane (E).
Limonene can be chemically characterized through acid-catalyzed hydration, where sulfuric acid (H₂SO₄) dissociates in water to form hydronium ions (H₃O⁺), conjugate acid, and conjugate base (HSO₄⁻), as shown in Fig. 4. Additionally, the bromination mechanism using hydrobromic acid (HBr) can be studied, as illustrated in Fig. 5. This demonstrates a non-conjugated diene with terminal and non-terminal carbon-carbon double bonds. The terminal double bond reacts preferentially to form product (C), while excessive HBr reacts with the non-terminal bond to yield 1-bromo-4-(2-bromopropan-2-yl)-1-methylcyclohexane (E).
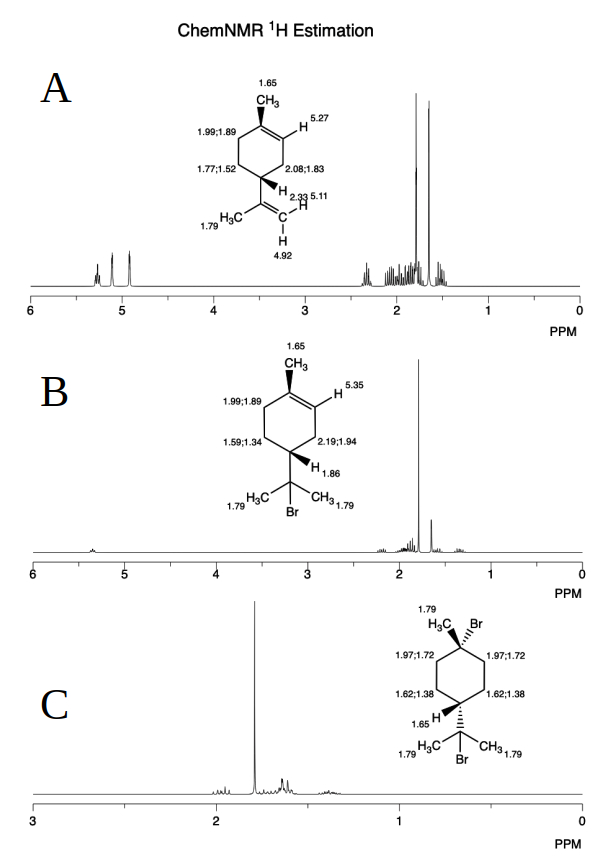
Figure 6: Estimated ¹H NMR spectra of limonene: (A) before reaction, and (B) after treatment with HBr on the terminal carbon double bond, and (C) on the non-terminal carbon double bond. The structures were optimized using Gaussian 9.0 and prediction structure converted to ChemDraw 20.1 for ¹H NMR spectra drawing.
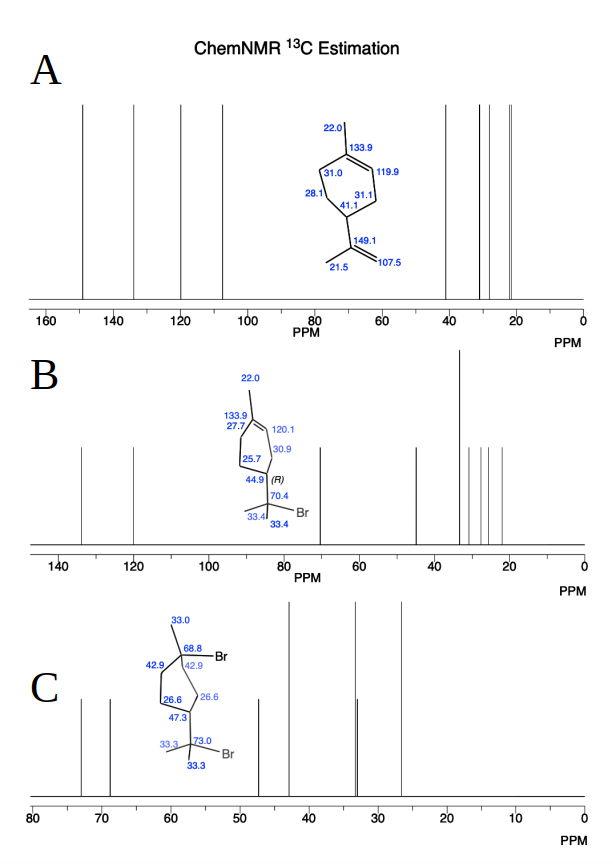
Figure 7: Estimated ¹³C NMR spectra of limonene: (A) before reaction, and (B) after treatment with HBr on the terminal carbon double bond, and (C) on the non-terminal carbon double bond. The structures were optimized using Gaussian 9.0 and prediction structure converted to ChemDraw 20.1 for ¹³C NMR spectra drawing.
Figures 6 and 7 show the results of the Gaussian calculation methodology, which combines quantum mechanical calculations to predict the NMR spectrum of limonene with high precision and determines the chemical shifts in ppm relative to TMS. For chemical structure optimization, the structure of limonene was optimized using Gaussian 09 [10], a widely used computational chemistry software. The optimization was performed using the MP2 (Møller-Plesset perturbation theory) method, which provides a good balance of accuracy and computational cost for ground-state calculations. The 6-31G basis set was employed, a moderate-sized basis set commonly used for such studies. The optimization included a frequency calculation (Opt/Frequency), ensuring that the structure corresponds to a true minimum on the potential energy surface (i.e., no imaginary frequencies).
For the prediction of ¹H and ¹³C NMR spectra, the GIAO (Gauge-Independent Atomic Orbital) method of calculation was used. GIAO is a standard approach for computing chemical shifts and is known for its reliability in reproducing experimental NMR spectra. This step was performed on the optimized structure to predict the ¹H and ¹³C NMR chemical shifts. Finally, the optimized structures were converted to ChemDraw 20.1 [11] to generate visual representations of the ¹H and ¹³C NMR spectra (see Figs. 6 and 7).
The steam distillation process effectively extracted limonene from orange peels, demonstrating its efficiency in isolating volatile, hydrophobic compounds. Combined with TLC, the method proved effective for isolating and analyzing limonene, with potential for optimization. TLC analysis showed a higher concentration of limonene in the organic layer, indicated by its higher Rf value, as the non-polar substance moved further on the polar TLC surface. Further purification yielded a sample with minimal impurities. While the 50:50 hexane and t-butyl methyl ether mixture worked for extraction, a toluene and ethanol mixture would likely provide better polarity for clearer separation.
The infrared (IR) spectrum provided a molecular fingerprint of limonene’s structure, and chemical reactions helped study its mechanism as a non-conjugated diene with terminal and non-terminal carbon-carbon double bonds.
Finally, the Gaussian methodology, which integrates quantum mechanical calculations with NMR prediction techniques, was employed to accurately estimate the NMR spectra of limonene.
We, the students of the Organic Chemistry course, would like to express our heartfelt gratitude to Professor JN for exceptional guidance and dedication. The expertise and encouragement provided have inspired us to deepen our understanding of the subject and explore new avenues in research.
We also extend our sincere thanks to the Natural Science Department at LaGuardia Community College for providing us with the resources and support that have been essential for our academic and research endeavors.
[1] Shang et al. (2022) - "Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes" [MDPI Antioxidants Journal] (https://www.mdpi.com/2076-3921/11/2/239)
[2] Frontiers in Nutrition (2022) - "Nutritional Composition and Bioactive Compounds of Citrus Byproducts" Frontiers in Nutrition, (https://www.frontiersin.org/articles/10.3389/fnut.2022.812108/full)
[3] Zhang et al. (2021) - "Extraction and Applications of Citrus Peel Bioactive Compounds" Processes Journal, MDPI (https://www.mdpi.com/2227-9717/10/6/1082)
[4] United States Department of Agriculture. (2022). Citrus: World Markets and Trade. Available online at: (https://usda.library.cornell.edu/concern/publications/w66343603?locale=en (accessed June 7, 2022))
[5] Saini, Ramesh Kumar; Nile, Shivraj Hariram; Park, Se Won. (2015). Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Research International, doi:10.1016/j.foodres.2015.07.047
[6] Shaw, P. E. Fresh Squeezed Orange Juice Odor: A Review. Crit. Rev. Food Sci. Nutr. 2008, 48 (7), 681–695. (https://doi.org/10.1080/10408390701638902)
[7] Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods. Int. J. Food Microbiol. 2004, 94 (3), 223–253. (https://doi.org/10.1016/j.ijfoodmicro.2004.03.008)
[8] Budavari S., (Ed.), The Merck index- An encyclopedia of chemical, drugs, and biologicals, 12th ed., Merck and Co., Inc., Whitehouse Station, NJ, 1996, p. 413
[9] Kenneth L. Williamson, Katherine M. Masters. 2010. Macroscale and Microscale Organic Experiments 6e. Chapter 6, p. 102
[10] Gaussian 09, Revision A.1, M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al. Gaussian, Inc., Wallingford CT, 2009
[11] ChemDraw 20.1, Perkin Elmer Informatic Inc. (1985-2021)