by Selassie Mawuko, Aminata Traore, Bibi-Sakeena Khemraj, and Anna Steto, and Dr. Richa Gupta
Tuberculosis (TB) in humans, caused by Mycobacterium tuberculosis, is a significant global health concern, resulting in high disease burden and mortality each year which together make it the world’s topmost infectious killer. The emergence of multidrug-resistant tuberculosis (MDR-TB) in recent decades has further exacerbated the present situation and warrants an urgent need to develop and investigate innovative treatment regimes. This study aims to assess the therapeutic potential of newly identified drug candidates, namely Bedaquiline, Delamanid, and Linezolid, in treating MDR-TB. We adopted a consolidated and integrated approach encompassing a thorough literature review, empirical experimental studies, multiple sequence alignments, and computational analyses, towards an in-depth analysis of the gene targets of these drugs and their efficacy in M. tuberculosis. Our findings indicate the significant potential of these drugs for tackling and treating drug-resistant TB, especially when synergized with prevailing first-line and second-line drugs. Notably, our results show a stark difference in the proteins and enzymes targeted by these drugs in mycobacteria versus those of the representative gut bacteria, providing a rationale for the minimal or mild side effects while administering these drugs in TB patients. Despite current infectious disease crises, including the COVID-19 disease, developing novel antibiotic treatments remains paramount against several serious bacterial pathogens. Our research offers a renewed perspective on enhancing therapeutic interventions by delving into drug combinatorial therapy against deadly infections, akin to those employed against drug-resistant tuberculosis.
Keywords: Multidrug-resistant tuberculosis (MDR-TB), Bedaquiline, Delamanid, Linezolid, Minimum Inhibitory Concentration (MIC), Phylogenetic Analysis, ATP synthase, Peptidyl transferase, Mycolic acid synthases, Synergistic drug combinations.
Tuberculosis (TB) is a severe infectious disease caused by the Gram-positive bacteria, Mycobacterium tuberculosis. For more than a century, TB has been a lingering global health challenge, transmitted mainly through the airborne route wherein infected individuals expel droplets or aerosols (containing the pathogenic bacteria) by coughing or sneezing that get inhaled by other nearby people leading to the spread of infection. In 2022, an estimated 10.6 million people fell ill with TB worldwide, of which 5.8 million were men, 3.5 million were women and 1.3 million were children; people with HIV infection accounted for 6.3% of the total TB patients (World Health Organization, 2023). Tuberculosis can have a profound and wide-ranging impact on the human body and is the leading cause of death in patients with HIV/AIDS and other immunocompromising conditions. In terms of prognosis of the disease, after infection with M. tuberculosis, about 5% of the infected individuals develop active TB exhibiting noticeable symptoms whereas the remaining 95% are asymptomatic latent TB cases. This infectious disease primarily targets the lungs, leading to a cascade of health issues. When M. tuberculosis enters the body and multiplies, it often forms nodules and tubercles in the lung tissue, leading to inflammation and damage. The immune system attempts to contain the infection by forming granulomas around these nodules, effectively walling off the bacteria (Ehlers & Schaible, 2013). Over time, this results in the characteristic symptoms of active TB, which include a persistent cough, fatigue, weight loss, fever, night sweats, and coughing of blood. However, M. tuberculosis doesn't confine itself to the respiratory system alone; it can spread throughout the body, affecting various other organs such as the bones, brain, kidneys, spleen, and lymph nodes, leading to a condition known as extrapulmonary tuberculosis (Baykan et al., 2022). In severe cases, TB can lead to life-threatening complications, and if left untreated, it can cause significant organ damage and ultimately even death. Beyond the physical toll of fatigue and health deterioration, TB also imposes a significant emotional and psychological burden. This stems from the stigma and isolation experienced by individuals with active disease, primarily driven by the fear of transmitting the contagious pathogen. Therefore, the effects of tuberculosis on our human civilization extend far beyond its immediate physical symptoms, affecting both the psychological and social well-being of those who are infected. Early diagnosis and proper treatment are crucial to mitigate the devastating impacts of the disease. In 2022, TB was the second leading infectious disease killer worldwide, after COVID-19. It was also the leading killer of people with HIV and a major cause of deaths related to antimicrobial resistance (World Health Organization, 2023).
The global burden of TB is compounded by the emergence of drug-resistant strains, particularly multidrug-resistant TB (MDR-TB). MDR-TB is characterized by resistance to potent TB treatment drugs such as isoniazid and rifampin, leading to treatment failure in many cases and prolonged duration of illness (Davidson et al., 2016). Noticeably, non-compliance or the misuse of drug combinations can accelerate the rise of MDR-TB, highlighting the critical importance of correct drug administration. The alarming growth of drug resistance in TB has ushered in new research studies directed toward the development of novel antimicrobial compounds and therapeutic strategies (Harris et al., 2014). Our research pivots on several promising drug candidates against MDR-TB, namely Bedaquiline, Delamanid, and Linezolid. In this paper, we further detail the molecular targets of these drugs and present the results of our comparative genomic and protein sequence analyses, which offer more insights into the therapeutic potential of these drugs. We also examined the efficacy, resistance profiles, and potential side effects of these drugs, to improve future patient treatment outcomes, notably with MDR-TB.
The cell wall structure of M. tuberculosis provides a protective barrier contributing to the pathogenesis of tuberculosis. First-line anti-TB drugs, like isoniazid and ethambutol, target cell wall biosynthesis. Other cellular processes have also emerged as promising targets for some anti-TB drugs, such as rifampin and fluoroquinolones (Riccardi et al., 2020). Recent studies and clinical trials have underlined the potential of a few novel drugs in treating drug-resistant TB. Unraveling the precise molecular mechanisms of these novel drugs can facilitate their optimal usage and predict potential resistance. Among the new drug candidates, as indicated in Fig. 1, Bedaquiline, a diarylquinoline, blocks the energy metabolism pathway by specifically inhibiting the essential mycobacterial ATP synthase enzyme, whereas Delamanid, a nitroimidazole derivative, disrupts unique aspects of the mycobacterial cell wall by inhibiting the synthesis of keto- and methoxy- mycolic acids, which are the key components of the cell wall (Khoshnood et al., 2021). Linezolid, an oxazolidinone, suppresses protein synthesis by binding to the bacterial amino acyl-tRNA initiation complex and blocks protein elongation by inhibiting the peptidyl transferase enzyme (Velez & Janech, 2010).
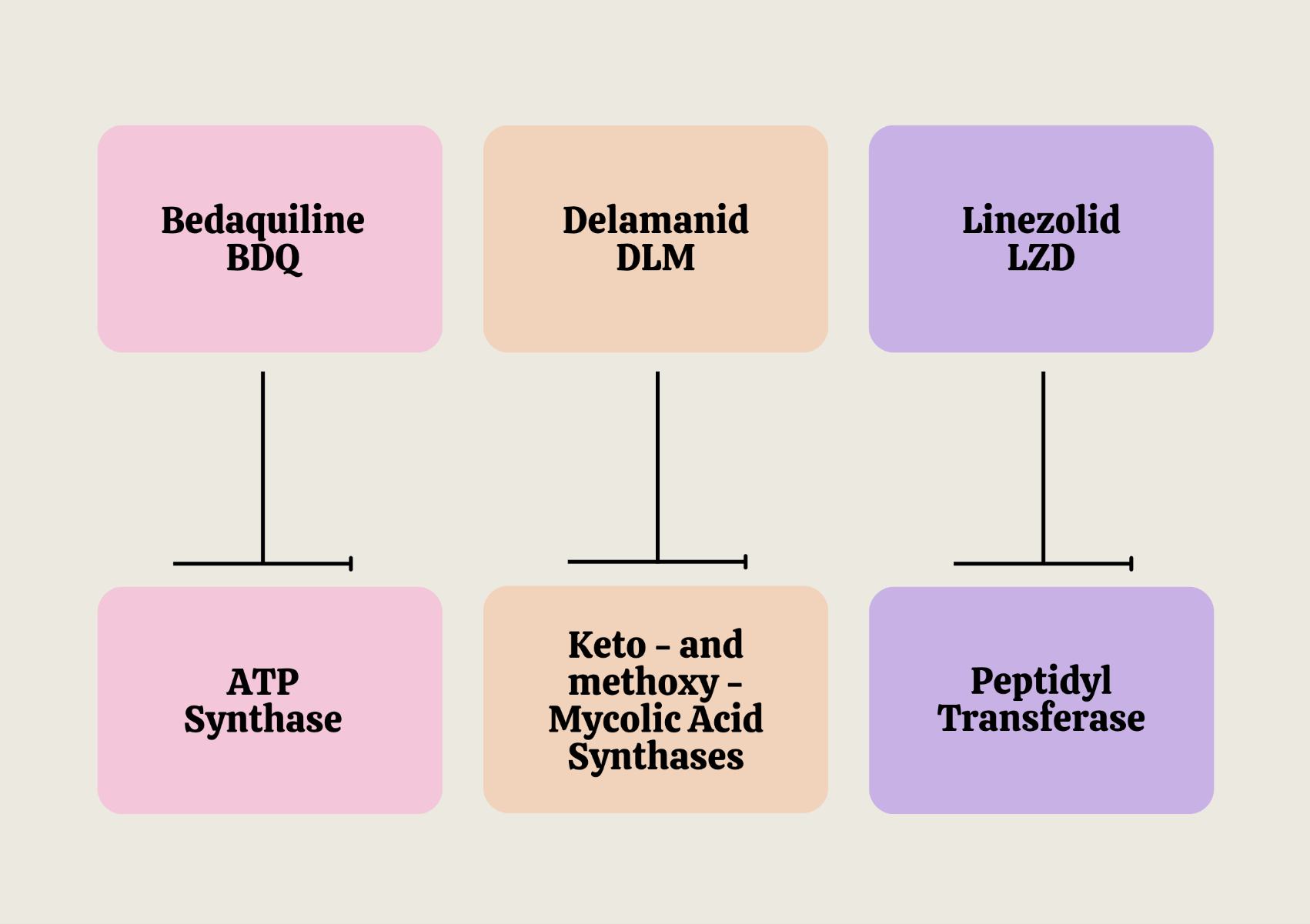
Figure 1: Molecular targets of Bedaquiline, Delamanid, and Linezolid in M. tuberculosis.
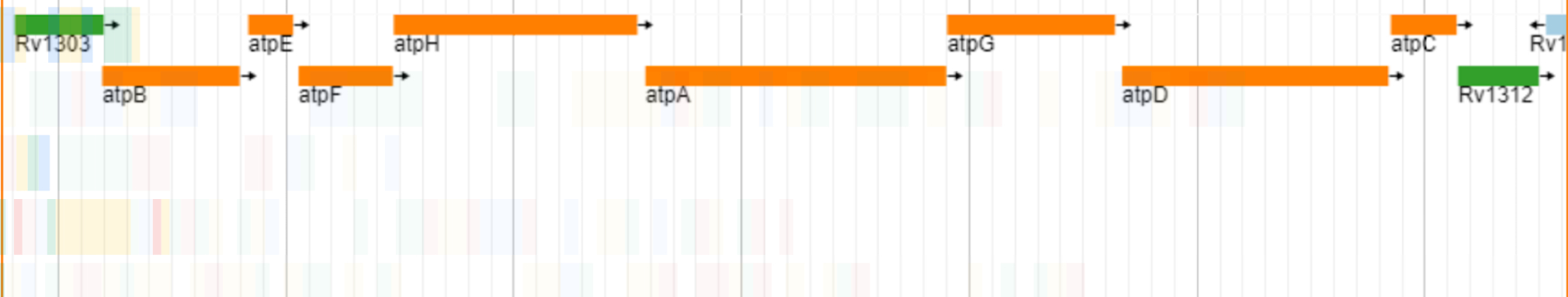
Our studies on the genomic architecture of the gene targets of the three novel anti-TB drugs revealed that all of them are organized in operonic arrangements with other neighboring genes in the M. tuberculosis chromosome, indicating common regulatory mechanisms for their expression. The target of Bedaquiline is the ATP Synthase C subunit, encoded by the atpE gene in M. tuberculosis, which plays a pivotal role in the bacterium's energy metabolism. The gene is 246 base pairs long, encodes for a protein consisting of 81 amino acids. As shown in Fig. 2A, the atpE gene is situated within an operon, co-localized with the genes for other subunits of the ATP synthase enzymatic complex that function in concert. Bedaquiline drug specifically inhibits the ATP Synthase C subunit and by targeting this essential component, it effectively impairs the survival and proliferation of mycobacteria.
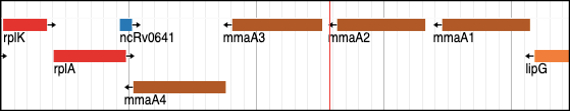
With regard to the mechanism of inhibition of Delamanid, the targeted bacterial enzymes are encoded by mmaA3 and mmaA4 genes and are involved in mycolic acid synthesis. The two genes are 882 base pairs and 906 base pairs long, respectively, and produce MmaA3 and MmaA4 proteins of 293 and 301 amino acids (Fig. 2B). MmaA4 is crucial in generating the hydroxymycolate precursor for synthesizing oxygenated mycolic acids. In the next step of the mycolic acid biosynthetic pathway, MmaA3 is tasked with O-methylating the hydroxymycolate precursor to produce methoxy-mycolates and keto-mycolic acids simultaneously (Alahari et al., 2009; Yuan & Barry, 1996). Delamanid weakens the bacterial cell wall by inhibiting mycolic acid synthesis, making the bacteria more vulnerable to the immune system and other anti-tuberculosis drugs. While the precise interactions with MmaA3 and MmaA4 may not be fully understood, delamanid disrupts mycolic acid production by likely influencing the function or expression of MmaA3 and MmaA4, ultimately leading to the death of the bacteria.
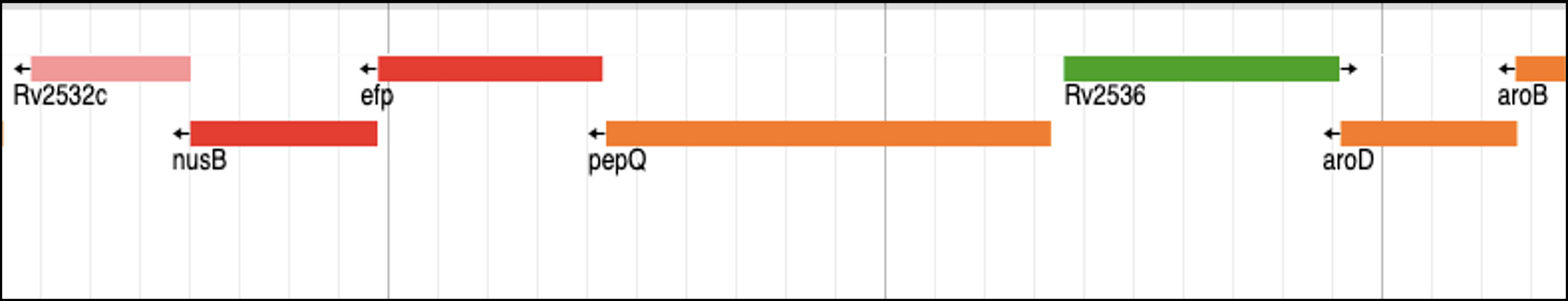
The efp gene found in M. tuberculosis chromosome is crucial for protein synthesis as it encodes the peptidyl transferase enzyme, which is essential for forming peptide bonds during translation. The efp gene is 564 base pairs long and encodes a protein consisting of 187 amino acids. Efp plays a critical role in facilitating the elongation of the growing peptide chain. Linezolid targets the aminoacyl-tRNA initiation complex and by binding to Efp, it disrupts the peptidyl transferase function, thereby effectively halting protein synthesis in the bacterium. This makes the drug particularly effective against replicating bacteria as it impedes the cellular ability to produce essential proteins for bacterial growth and survival. As shown in Fig. 2C, in the case of M. tuberculosis, the efp gene is located in the same operon as nusB and pepQ genes. NusB is involved in regulating transcription and antitermination processes, vital for proper RNA synthesis and processing. On the other hand, PepQ is a cytoplasmic peptidase responsible for peptide metabolism. Together, the three proteins contribute to important aspects of gene regulation and expression.
Figure 2: Genomic organization of the novel drug targets in M. tuberculosis: (A) Bedaquiline target, the atpE gene, which encodes for the ATP Synthase C subunit; (B) Delamanid targets, mmaA4 and mmaA3, which encode for Keto- and methoxy-mycolic acid synthases, respectively; and (C) Linezolid target, the efp gene, which encodes for peptidyl transferase enzyme.
(A)
(B)
(C)
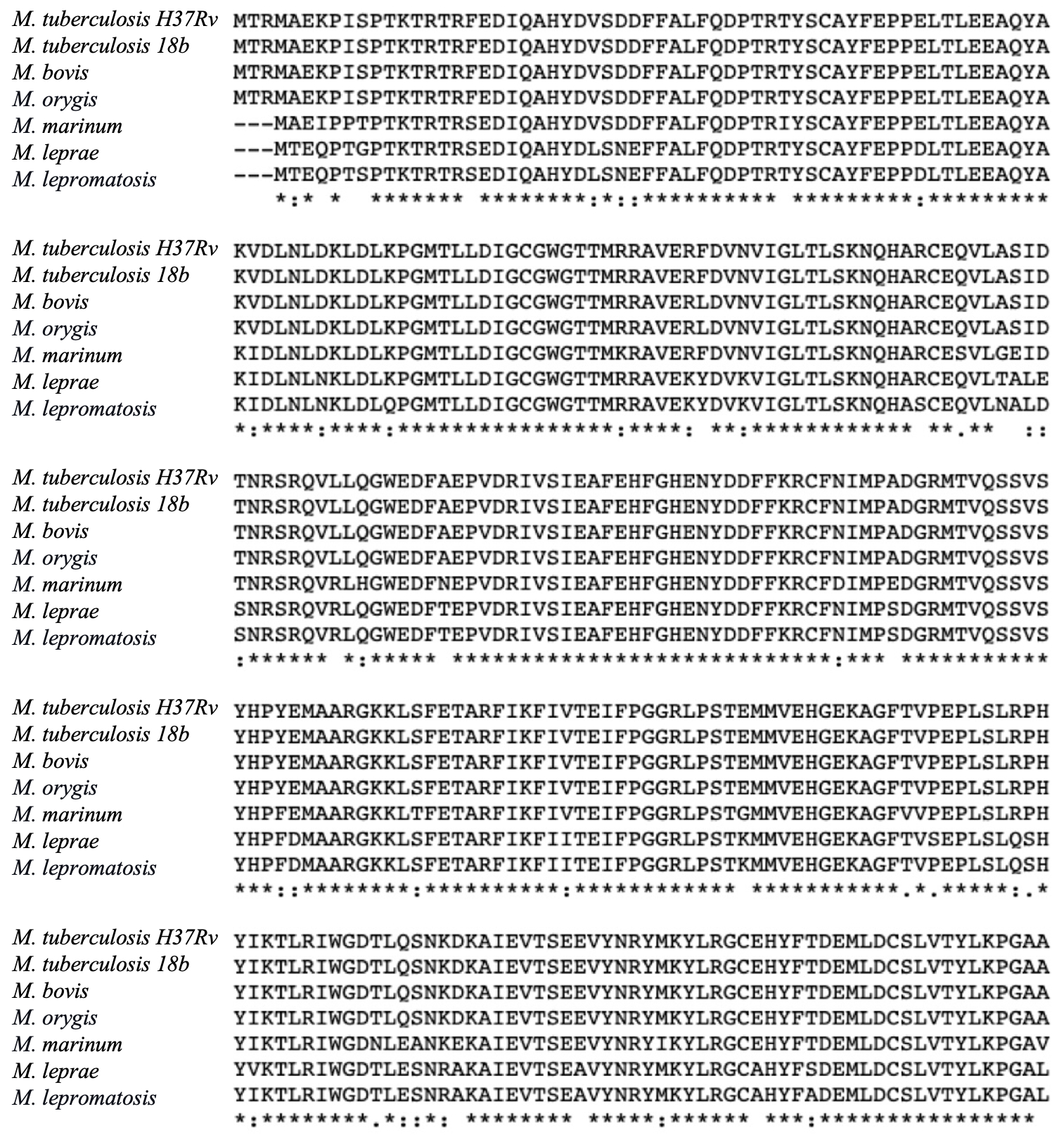
We next investigated the conservation of the drug targets in the Mycobacterium genus, by conducting multiple sequence alignment studies. The AtpE proteins, targeted by Bedaquiline, showed a high degree of conservation across the investigated mycobacterial species, with a mean percentage identity of 79.07% (68 identical amino acid and 7 similar amino acid positions) (see Fig. 3A). This finding suggests that Bedaquiline may have strong efficacy against different mycobacterial species. Similarly, the MmaA4 and MmaA3 enzymes, targeted by Delamanid, exhibited mean percentage identities of 90.03% and 87.03%, respectively, indicating their high degree of conservation among mycobacterial species (Fig. 3B and 3C). The mycobacterial Efp proteins, targeted by Linezolid, demonstrated a high mean percentage identity of 93.04% as well (Fig. 3D). These findings highlight the potential of these three drugs as effective therapeutic interventions against a wide range of mycobacterial infections. Furthermore, the conservation of these drug targets highlights their essential roles in mycobacterial physiology and survival, making them attractive candidates for the development of other new antimicrobial agents.
Figure 3: Multiple sequence alignment of the novel mycobacterial drug targets: (A) AtpE proteins were compared and analyzed across different mycobacterial species, i.e. M. tuberculosis H37Rv, M. smegmatis, M. bovis, M. abscessus, M. leprae, M. lepromatosis, M. tuberculosis 18b, and M. marinum; (B) Comparison of MmaA4 proteins from different species of mycobacteria, i.e. M. tuberculosis H37Rv, M. tuberculosis 18b, M. bovis, M. orygis, M. marinum, M. leprae, M. lepromatosis;(C) Comparison of MmaA3 proteins from different species of mycobacteria, i.e. M. bovis, M. tuberculosis H37Rv, M. orygis, M. tuberculosis 18b, M. marinum; and (D) Analysis of Efp protein sequences from different mycobacterial species, i.e. M. tuberculosis, M. bovis, M. leprae, M. marinum, M. smegmatis.
(A)

(B)
(C)
(D)

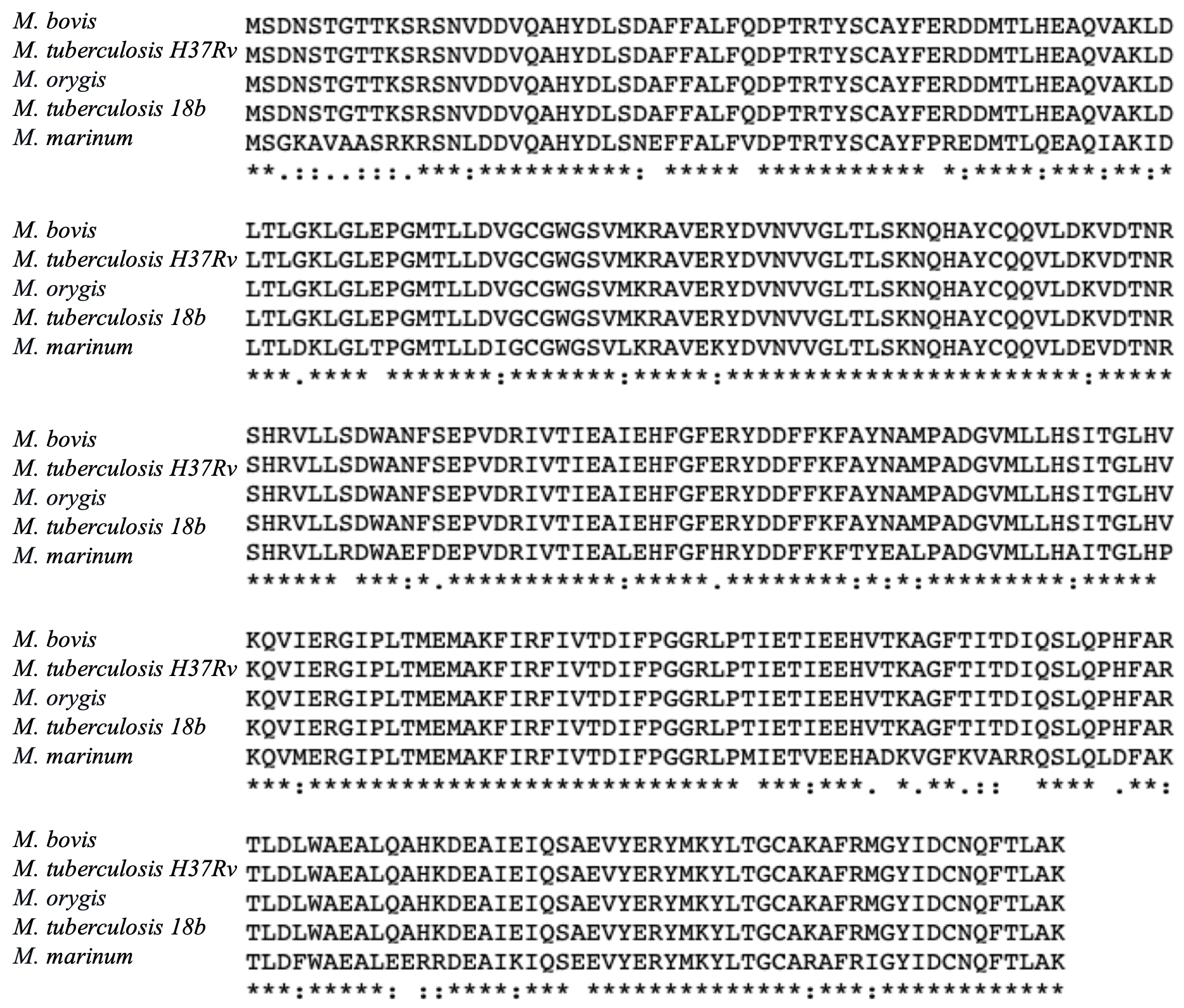
To evaluate the optimal potential of a drug for treating a disease, it is important to determine that the targeted protein of the pathogenic organism is specifically inhibited, and the drug will not affect the activity of similar proteins of our gut microbiota for minimal side effects. With this rationale, we conducted multiple sequence alignment of the new mycobacterial drug targets (i.e. AtpE and Efp) with their corresponding proteins encoded by different classes of bacteria commonly found in our gut, such as Clostridium butyricum, Faecalibacterium prausnitzii, Blautia hansenii, and Escherichia coli (please note: MmaA3 and MmaA4 are specific to mycobacteria and absent in these gut bacteria). Investigation of the protein homology between AtpE sequences of various mycobacterial species with those of the gut bacteria indicated a mean percentage identity of only 8.5%, with 8 identical and 22 similar amino acid positions (see Fig. 4A). On the other hand, comparison of the Efp protein sequences revealed a mean percentage identity of 45.8%, with the M. tuberculosis protein sharing homology of 46.5% with C. butyricum, 50.8% with B. hansenii, 44.3% with F. prausnitzii, and 41.7% with E. coli (see Fig. 4B). Strikingly, both the drug targets are highly conserved within mycobacteria (80-90% identical, see Fig. 3) but are remarkably different to the respective proteins of our gut clostridia (i.e. C. butyricum, B. hansenii, F. prausnitzii) and gammaproteobacteria (E. coli). Therefore, our results support the use of Bedaquiline and Linezolid as effective drugs against diseases caused by mycobacteria.
Figure 4: Multiple sequence alignment of the drug target proteins from various mycobacterial species versus gut bacteria. (A) AtpE proteins were compared and analyzed from M. tuberculosis H37Rv, M. smegmatis, M. bovis, M. abscessus, M. leprae, M. tuberculosis 18b, M. marinum, C. butyricum, F. prausnitzii, B. hansenii, and E. coli; and (B) Analysis of Efp protein sequences from M. tuberculosis H37Rv, M. bovis, M. leprae, M. marinum, M. smegmatis, C. butyricum, F. prausnitzii, B. hansenii, and E. coli.
(A)

(B)

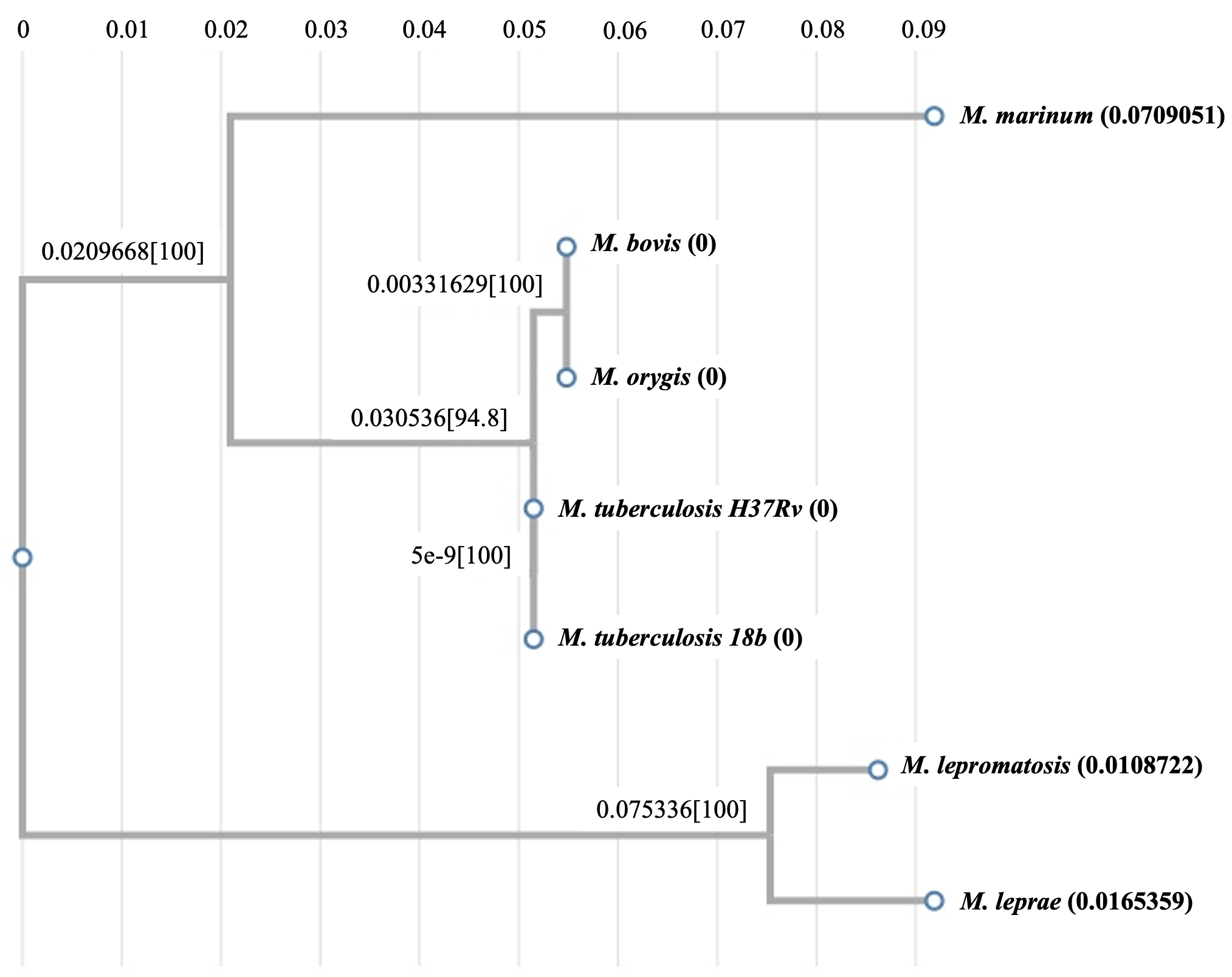
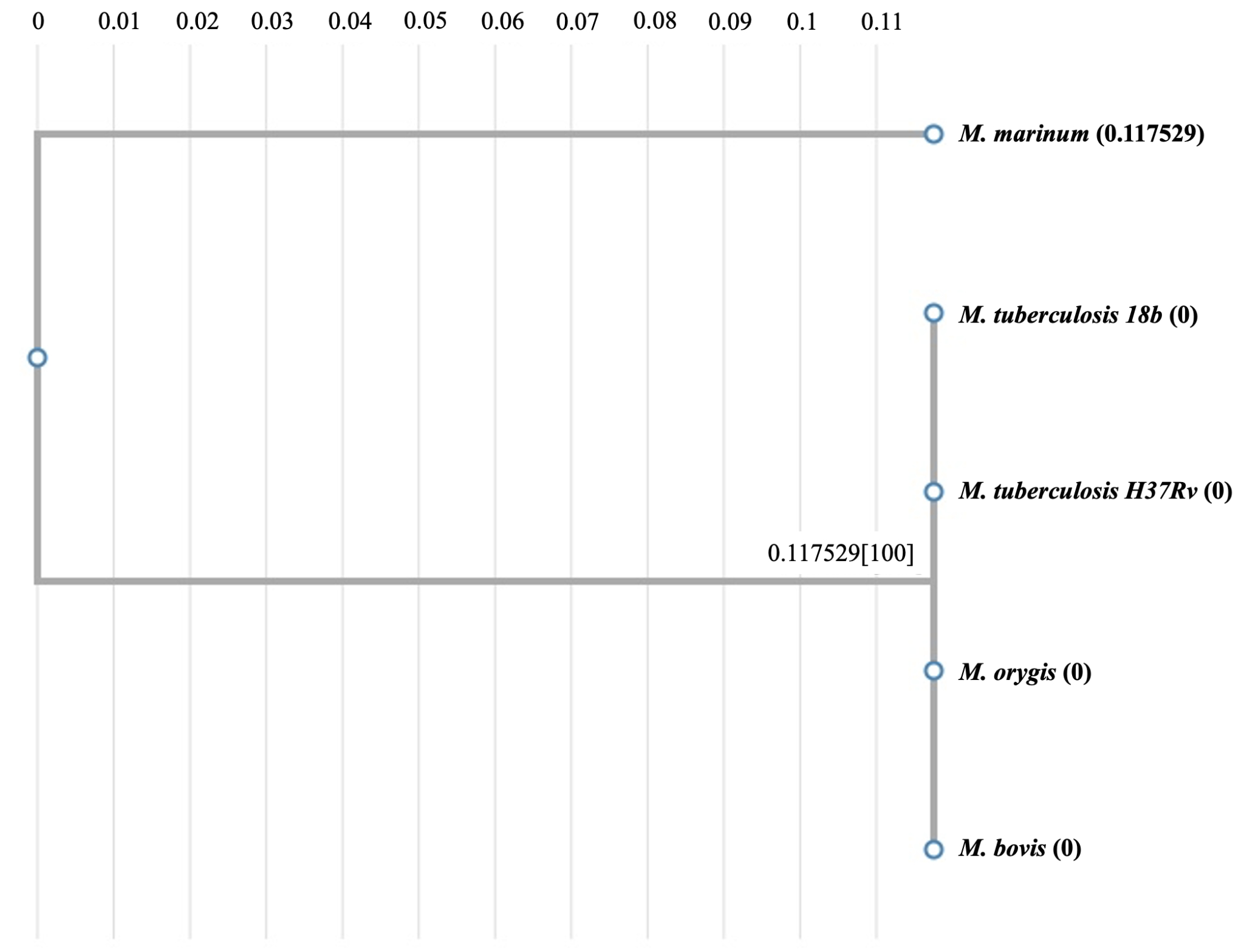
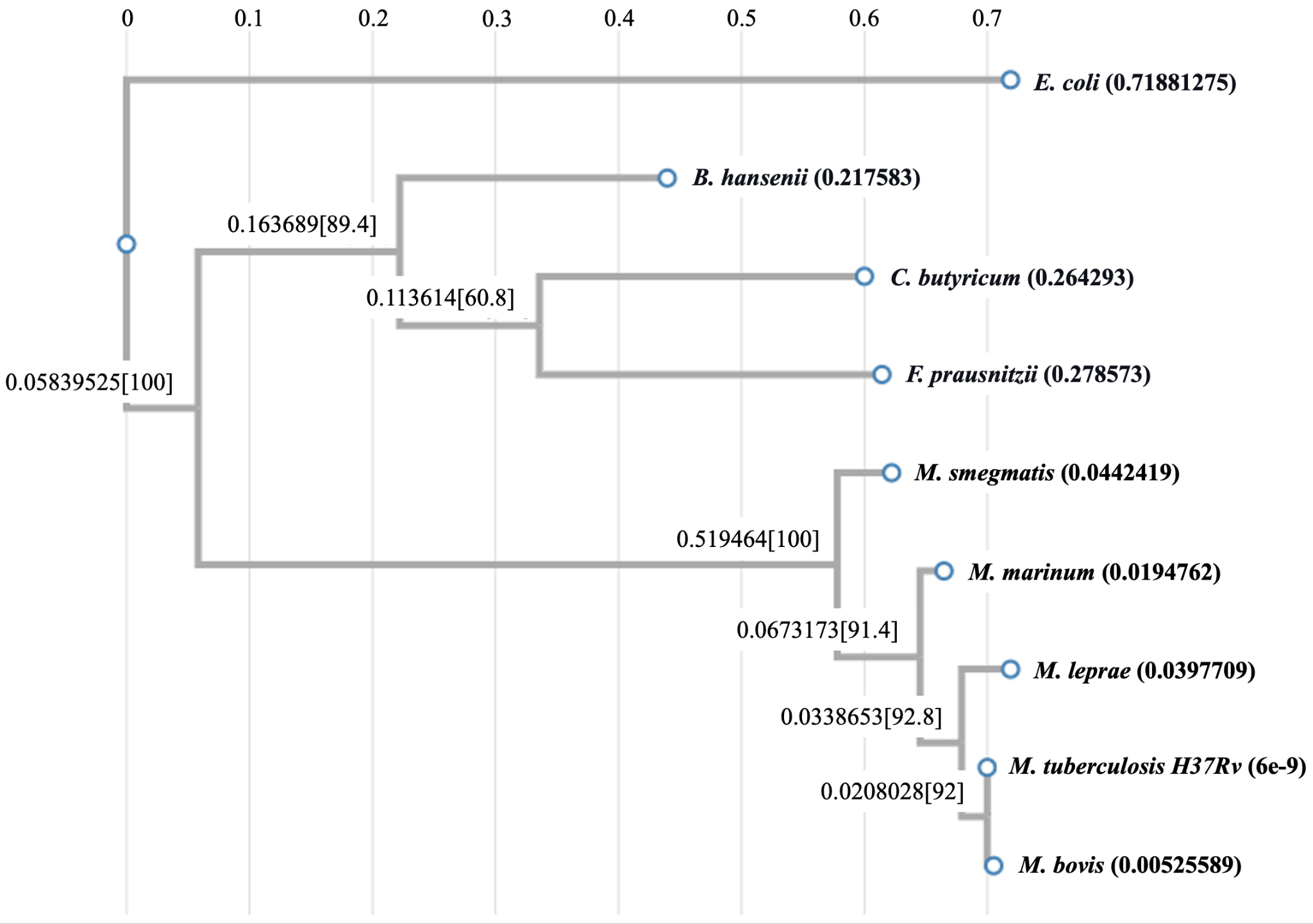
To gain insights into the evolution of the drug target proteins in different classes of bacteria, we constructed their phylogenetic trees. As can be seen in Fig. 5A, all the mycobacterial AtpE sequences form a close cluster indicating their concomitant evolution. In contrast, the AtpE sequences from C. butyricum, F. prausnitzii, and B. hansenii form a separate distant cluster and seem to have evolved at a different time in history. Interestingly, AtpE protein of the Gram-negative bacterium E. coli falls closer to the group of Gram-positive mycobacterial proteins than the group of Clostridial proteins, hinting at its possible intermediate evolution.
Regarding the MmaA3 and MmaA4 sequences, which are found only in the mycobacterial species (and not in these gut bacteria), both M. tuberculosis enzymes are most homologous to their corresponding M. bovis counterparts, followed by other mycobacteria such as M. marinum and M. leprae (see Fig. 5B and 5C). Our exploration of the evolution of bacterial Efp proteins revealed that the E. coli Efp stands out as a distantly segregated protein among all sequences from the evolutionary perspective, and the mycobacterial and clostridial sequences form two separate relatively closer clusters suggesting their possible divergent evolution (see Fig. 5D).
Figure 5: Analysis of evolutionary relationships. (A) Phylogenetic tree of AtpE proteins from various mycobacterial species versus different gut bacteria; (B) Phylogenetic tree of MmaA4 sequences from different mycobacterial species; (C) Phylogenetic tree of MmaA3 sequences from different mycobacterial species; and (D) Phylogenetic tree of Efp proteins from various mycobacterial species versus different gut bacteria.
(A)

(B)
(C)
(D)
In the fight against TB and other mycobacterial diseases, the efficacy of several antimicrobial drugs is critical. Particularly, TB is a chronic worldwide health issue. Our computational studies on the new drug target genes indicated that these genes are highly conserved among several mycobacterial genomes, including both pathogenic and non-pathogenic species. This led us to next conduct some empirical growth inhibition studies with the commonly known and available drugs against mycobacteria. We specifically analyzed how some known antibiotics affect the survival of non-pathogenic and pathogenic mycobacteria at different doses. M. smegmatis is a non-pathogenic saprophyte which can be grown easily in a biosafety level 1 laboratory and has a similar cell wall structure as M. tuberculosis and other pathogenic mycobacteria, with an added advantage of a faster doubling time; this prompted us to use M. smegmatis for comparative drug efficacy analyses in our studies. The minimal inhibitory concentrations (MICs) of rifampin, ciprofloxacin, and kanamycin against M. tuberculosis have long been reported in the literature.
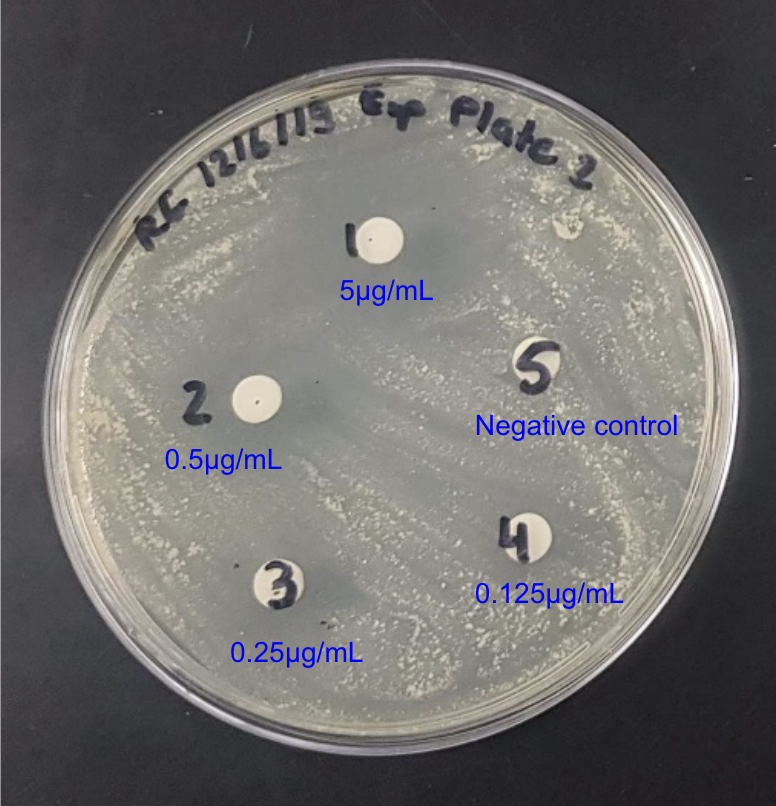
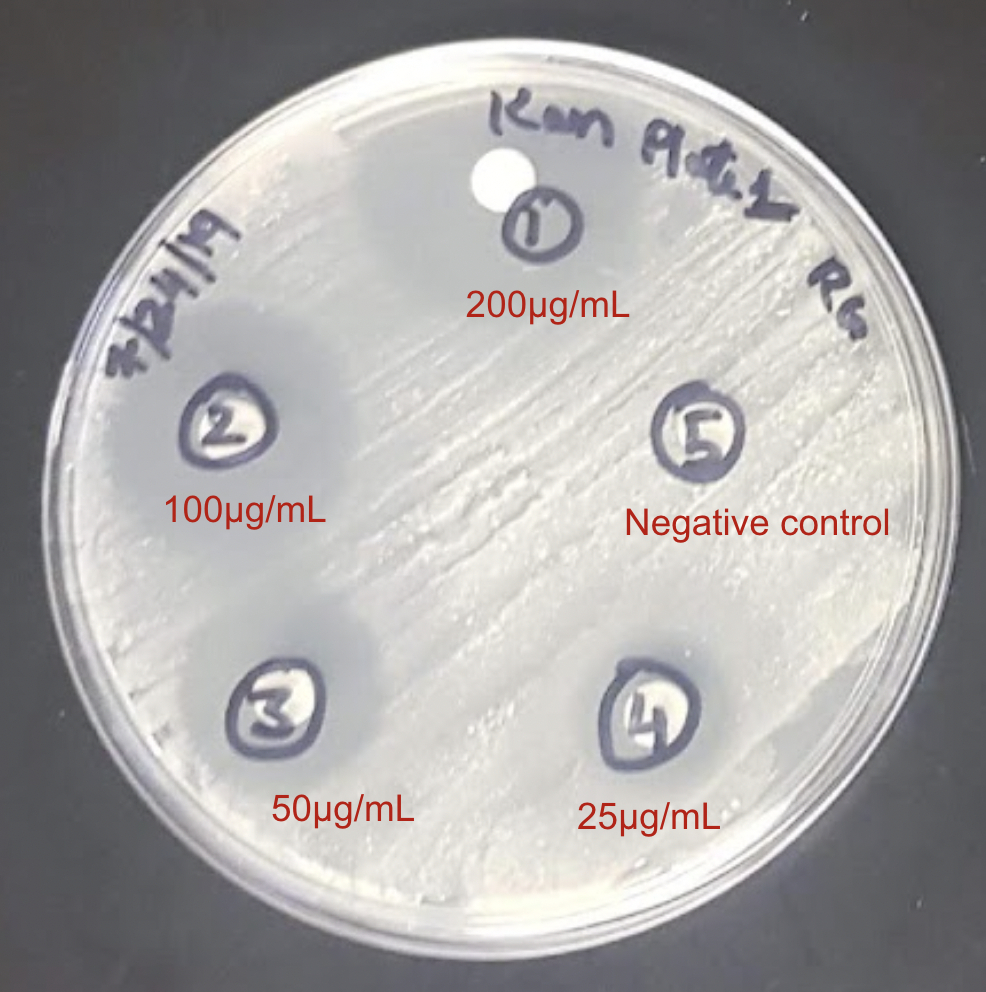
The effectiveness of rifampin as an anti-tuberculosis drug is well-established, as demonstrated by its capacity to impede M. tuberculosis growth by blocking cellular transcription with the reported MIC value of ~1 μg/ml (Zhang et al., 2019). The results of our growth inhibition studies indicated that rifampin inhibited M. smegmatis growth within a similar dose range of 20 to 200 μg/ml (see Fig. 6A). Similarly, the commonly used antibiotic, ciprofloxacin, significantly inhibited the growth of M. smegmatis at a dose of 0.25 μg/ml and beyond (see Fig. 6B)), in close accordance with its reported MIC range of 0.125 to 1 μg/ml against M. tuberculosis (Yilmaz et al., 2018). Additionally, the antimicrobial drug, kanamycin, showed strong growth inhibitory effects against M. smegmatis at doses ≥ 25 μg/ml, which is fairly consistent with its MIC range of 2 to 3 μg/ml against M. tuberculosis (Dijkstra et al., 2018). Our previous computational studies revealed that the target proteins of the new drugs are highly conserved among various mycobacterial species. In line with this, three antibacterial compounds demonstrated expected inhibitory outcomes in our experimental growth inhibition studies with the known drugs by effectively inhibiting M. smegmatis at doses close to the reported MIC values for M. tuberculosis (see Table 1). These results demonstrate the powerful use of M. smegmatis as a model system for examining the effectiveness of antibiotics against mycobacteria and the possibility of translating these inhibitors into effective treatment options against multi-drug resistant TB, in combination with other new drug candidates. Recognizing the dose-dependent inhibitory effects of new drug candidates in the future will help to optimize treatment plans and support ongoing efforts to control TB.
Figure 6: Disk-diffusion assays of exponentially grown M. smegmatis cultures with the indicated doses of: (A) Rifampin, (B) Ciprofloxacin, and (C) Kanamycin. Representative plate pictures are shown.
(A)

(B)
(C)
| Antibiotic tested | Growth Inhibitory Dose Range (for M. smegmatis) | MIC Reported in Literature (for M. tuberculosis) |
|---|---|---|
| Rifampin | 20 - 200 μg/ml | 1 μg/ml |
| Ciprofloxacin | 0.25 - 5 μg/ml | 0.125 to 1 μg/ml |
| Kanamycin | 25 - 200 μg/ml | 2 to 3 μg/ml |
This comprehensive study integrating genomic and bioinformatic analyses with experimental approaches provides valuable insights into the three new anti-TB drug candidates: Bedaquiline, Delamanid and Linezolid. Analysis of the targeted proteins, AtpE, MmaA3, MmaA4 and Efp, reveals strong selective pressure and functional conservation of these proteins in various mycobacterial species, implying their critical role in the physiology of these bacteria.
Our results reveal the genomic architecture of these promising drug targets. Intriguingly, the genes encoding the targets of these drugs were found to be organized in operonic arrangements in M. tuberculosis, suggesting coordinated transcriptional regulation. The presence of operons is a common feature observed in prokaryotic genomes and this organizational structure involves the clustering of functionally related genes into a single transcriptional unit, allowing for their simultaneous expression and regulation. For example, the atpE gene (encoding the ATP synthase C subunit targeted by Bedaquiline) is co-localized with other genes encoding different subunits of the ATP synthase complex. These genes together make up the atp operon, which is regulated by the transcriptional repressor ATPaseR (Hards et al., 2015). Similarly, the mmaA3 and mmaA4 genes (targeted by Delamanid) appear to be organized within an operon responsible for mycolic acid biosynthesis, along with other related genes, such as mmaA1 and mmaA2, and are influenced by the transcriptional regulators MmaR and FasR (Alahari et al., 2009; Mondino et al., 2013). Furthermore, the efp gene (encoding the peptidyl transferase targeted by Linezolid) is present in an operon with nusB and pepQ genes, which are involved in transcriptional regulation and peptide metabolism, respectively (Ushtanit et al., 2021). This operonic arrangement facilitates the synchronized expression of these critical genes, ensuring the proper stoichiometry and timing of their protein products, and such intricate regulatory mechanisms are essential for the survival and pathogenicity of M. tuberculosis.
Sequence analyses revealed a high degree of conservation for the drug targets (AtpE, MmaA3, MmaA4, Efp) across various Mycobacterium species (~80-90%) but significantly lower similarity to their homologs in gut bacteria like E. coli and Clostridium sp., indicating potential for selective efficacy of these new drugs against mycobacteria while minimizing gut microbiome disruption and the associated gastrointestinal side effects compared to other broad-spectrum antibiotics. In accordance with this, some clinical studies have demonstrated considerable potency of these drugs. For instance, Bedaquiline helped in achieving 2-fold higher culture conversion rates versus standard therapy in MDR-TB patients (Diacon et al., 2014), and Delamanid showed promising results in Phase 3 trials, significantly improving sputum culture conversion and presented only mild side effects such as nausea, vomiting, and abdominal pain (Gler et al., 2012; Zou et al., 2023). In the case of Linezolid too, upon dosage optimization to 600 mg/day, 91% favorable outcomes with reduced toxicity among patients enrolled in the study were achieved (Conradie et al., 2022). Clinical studies have especially underscored the potency of Bedaquiline, when combined with other anti-TB drugs. A recent study involving 2731 patients with MDR/XDR-TB infections across 14 countries demonstrated promising outcomes for the concomitant use of Bedaquiline and Delamanid alongside other second-line anti-TB medications. Among the 472 patients (17.3%) who received the concomitant Bedaquiline and Delamanid treatment, 78% responded favorably to this treatment strategy, highlighting its potential as a safe and effective alternative for patients infected with drug-resistant TB strains or those who cannot tolerate other therapies. However, these studies noted that in some patient ECGs, there was a risk of QT interval prolongation, which necessitates careful ECG monitoring of patients (Huerga et al., 2022). These findings underscore the importance of continued research and clinical trials to optimize the use of novel drugs and improve patient outcomes in the fight against MDR/XDR-TB. Until other new potent drug candidates emerge, combinatorial trials like the bedaquiline-pretomanid-linezolid (BPaL) regimen hold promise for shorter, more effective treatment against highly drug-resistant TB (Conradie et al., 2022). Noticeably, the low resistance profile of Delamanid positions it as a valuable asset against MDR and XDR-TB strains. Given the effectiveness and reduced side effects at optimized dosages, we advocate for further combinatorial trials of Linezolid and Delamanid. Such trials could pave the way for novel therapeutic regimens that are more tolerable and effective against MDR-TB, based on the need of individual cases.
Another drug candidate, Verapamil, possesses untapped potential as a synergistic drug to improve the efficacy of other antibiotics by inhibiting efflux pumps in the mycobacterial membrane. It is a calcium ion channel blocker and inhibits efflux pumps by reducing the transmembrane potential (Adams et al., 2014). Verapamil is suggested as a complement to other anti-mycobacterial medications for enhanced therapeutic results. In vitro drug susceptibility testing of these new drugs has also been performed on M. tuberculosis strains in both planktonic and biofilm growth models. Bedaquiline exhibited the MIC of 0.25 μg/ml against planktonic cells (Adams et al., 2014), and Verapamil displayed synergistic effects with isoniazid, reducing its MIC by 4-fold (Koul et al., 2014). MIC of Linezolid was elevated to 16 μg/ml against biofilm cells, indicating reduced penetration.
The increasing prevalence of TB cases resistant to commonly used drugs like Isoniazid and Rifampin highlights the urgent need for new anti-mycobacterial drugs and antibiotics. Recent research suggests that combining first-line antibiotics with second-line antibiotics can reduce treatment duration for TB patients and may aid in treating MDR-TB to some extent. While promising new compounds have been identified as potential drug candidates, detailed bacterial susceptibility studies and clinical trials are essential to validate their efficacy against Mycobacterium species and address the growing concern of antibiotic resistance. In our growth inhibition experiments, we found that certain antibiotics, particularly ciprofloxacin, showed significant and reproducible dose-dependent inhibitory effects on mycobacterial growth, similar to those reported previously for M. tuberculosis. The use of M. smegmatis as a model organism in our experimental procedures further supports the validity of these findings, as M. smegmatis shares substantial genomic similarity with M. tuberculosis (~ 75%), making it an excellent surrogate for assessing drug efficacy. M. smegmatis is also faster growing and non-pathogenic, which eliminates the clinical health risks associated with working with an infectious agent. Furthermore, many of the virulence factors and mechanisms employed by M. tuberculosis are conserved in M. smegmatis and the two bacteria have similar cell wall composition, which is a key aspect in the pathogenesis of TB. To combat drug resistance, we propose exploring combinations of these new drugs with isoniazid, rifampin and ciprofloxacin. By combining multiple antibiotics with different mechanisms of action, we can enhance treatment options, shorten duration and overcome resistance issues. However, further research is needed to optimize these combination therapies and evaluate their clinical effectiveness.
Overall, this study identified and characterized next-generation anti-TB agents in the face of a pressing global challenge of drug-resistant TB. Further research is warranted to explore their long-term efficacy, detailed pharmacokinetic, toxicity and safety profiles, as well as their potential synergistic effects in combination therapy regimens in animal models of TB infection and human trials, in order to translate findings into viable treatment options and guide lead optimization efforts. Analogs of these drugs can be designed to further enhance potency and selectivity, leveraging the sequence analysis insights. Structure-activity relationship studies will also help to further the understanding of molecular mechanisms underlying drug action.
Importantly, consolidated strategies are needed to mitigate antitubercular drug resistance development. Novel genomic and proteomic techniques like transcriptomics and metabolomics can provide systems-level insights into drug effects and mycobacterial responses, and continued basic research can help to identify the genetic determinants and/or other involved cellular mechanisms leading to the emergence of resistant strains. Since significant challenges remain, anti-TB drug development and evolution of therapeutic strategies should be accelerated. Global partnerships and unremitting efforts are critical to winning the long battle against this ancient deadly disease and combating the threat of drug resistance. Implementing the novel therapeutic approaches in resource-limited settings, where TB is the most prevalent is critical, and can face additional challenges. The high cost of these drugs, limited availability, and the need for advanced diagnostic tools to detect drug-resistant strains may hinder their widespread use (Naidoo et al., 2023). Strengthening healthcare infrastructure, improving access to drugs and drug compliance, and developing affordable, rapid, and reliable diagnostic tests are crucial for the successful implementation of these novel therapies in resource-constrained environments. Cross-border collaborations and funding support will be essential to address these challenges and ensure equitable access to these life-saving treatments.
Comparative genomic organization and protein sequences analyses of the drug target genes (atpE, mmaA3, mmaA4, and efp) were conducted using various bioinformatic databases and applications, as listed below.
Mycobrowser: a genomic and proteomic data repository of different mycobacterial species.
GenBank: NIH genetic sequence database, an annotated collection of all publicly available DNA and protein sequences from different organisms.
CLUSTALW: for performing multiple sequence alignments and determining phylogenetic relationships.
M. smegmatis cultures were revived and grown at 37°C with constant shaking at 150 rpm, as described previously (Gupta et al., 2015). To conduct growth inhibition studies, disk diffusion assays were performed using Mueller Hinton (MH) medium. Logarithmic phase grown cultures were evenly spread on the MH agar plates and disk diffusion assays were performed using serial dilutions of different compounds. DMSO or water was included as the negative control on each plate, depending on the antibiotic. All experiments were performed in duplicates and repeated two or three times. Zones of inhibition were measured to determine the efficacy of compounds for growth inhibition. Wherever indicated, MIC is defined as the minimum dose of the compound at which 90% of the bacterial growth was inhibited.
This research was supported by CUNY Research Foundation, NIH Bridges grant 5T34GM137858-04 (NIH NIGMS), and CUNY Research Scholars Program (CRSP). Dr. Richa Gupta served as the Principal Investigator and Research Mentor, and is the recipient of the Cycle 48 PSC-CUNY research award and Community College Research Grant (CCRG); Selassie Mawuko, Aminata Traore, and Bibi- Sakeena Khemraj were supported by the NIH Bridges Undergraduate Research Scholarship; and Anna Steto was supported by the CRSP Undergraduate Research Scholarship.
[1] Chaisson, R. E., Frick, M., & Nahid, P. (2022). The scientific response to TB - the other deadly global health emergency. International Journal of Tuberculosis and Lung Disease, 26(3), 186-189. https://doi.org/10.5588/ijtld.21.0734
[2] World Health Organization. (2023, April 21). Tuberculosis. Retrieved May 20, 2023. https://www.who.int/news-room/fact-sheets/detail/tuberculosis
[3] Wu, K. J., Boutte, C. C., Ioerger, T. R., & Rubin, E. J. (2019). Mycobacterium smegmatis HtrA Blocks the Toxic Activity of a Putative Cell Wall Amidase. Cell Reports, 27(8), 2468-2479.e3. https://doi.org/10.1016/j.celrep.2018.12.063
[4] McGaha, S. M., & Champney, W. S. (2007). Hygromycin B inhibition of protein synthesis and ribosome biogenesis in Escherichia coli. Antimicrobial Agents and Chemotherapy, 51(2), 591-596. https://doi.org/10.1128/AAC.01116-06
[5] Velez, J. C., & Janech, M. G. (2010). A case of lactic acidosis induced by linezolid. Nature Reviews Nephrology, 6(4), 236-242. https://doi.org/10.1038/nrneph.2010.20
[6] Khoshnood, T., et al. (2021). Mechanism of action, resistance, synergism, and clinical implications of delamanid against multidrug-resistant Mycobacterium tuberculosis. Frontiers in Microbiology, 12, 717045. https://doi.org/10.3389/fmicb.2021.717045
[7] Houben, R. M. G. J., & Dodd, P. J. (2016). The global burden of latent tuberculosis infection: a re-estimation using mathematical modeling. PLOS Medicine, 13(10), e1002152. https://doi.org/10.1371/journal.pmed.1002152
[8] Centers for Disease Control and Prevention. (2022, May 4). Core Curriculum on Tuberculosis (2000).
[9] Falzon, D., et al. (2017). World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. European Respiratory Journal, 49(3), 1602308. https://doi.org/10.1183/13993003.02308-2016
[10] Langendam, M. W., et al. (2017). Adverse events after first-line anti-tuberculosis drug regimens: A systematic review and meta-analysis. International Journal of Tuberculosis and Lung Disease, 21(10), 1099-110.
[11] Sotgiu, G., et al. (2012). Efficacy, safety and tolerability of linezolid-containing regimens in treating MDR-TB and XDR-TB: Systematic review and meta-analysis. European Respiratory Journal, 40(6), 1430-1442. https://doi.org/10.1183/09031936.00022912
[12] Chatterjee, D. (1997). The mycobacterial cell wall: Structure, biosynthesis and sites of drug action. Current Opinion in Chemical Biology, 1(4), 579-588. https://doi.org/10.1016/S1367-5931(97)80045-2
[13] Sarathy, J. P., Lee, E., & Dartois, V. (2017). Drug approaches inhibiting mycobacterial cell wall-related processes. Current Topics in Microbiology and Immunology, 400, 155-175.
[14] Koul, A., Vranckx, L., Dhar, N., et al. (2014). Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nature Communications, 5, 3369. https://doi.org/10.1038/ncomms4369
[15] Almeida, D., Ioerger, T., Tyagi, S., Li, S. Y., Mdluli, K., Andries, K., Grosset, J., Sacchettini, J. C., & Nuermberger, E. (2016). Mutations in pepQ confer low-level resistance to bedaquiline and clofazimine in Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy, 60(8), 4590-4599. https://doi.org/10.1128/AAC.00753-16
[16] Gallo, J., Vaněk, J., Dvorská, L., et al. (2017). Structure of rRNA methyltransferase EfmM from Enterococcus faecium bound to S-adenosylhomocysteine and a sulfonamide inhibitor: Implications for the mechanism and drug discovery. Nucleic Acids Research, 45(22), 12908-12918. https://doi.org/10.1093/nar/gkx1047
[17] Adams, K. N., Szumowski, J. D., & Ramakrishnan, L. (2014). Verapamil and its metabolite norverapamil inhibit macrophage-induced, bacterial efflux pump-dependent tolerance to multiple anti-tubercular drugs. Journal of Infectious Diseases, 210(3), 456-466. https://doi.org/10.1093/infdis/jiu104
[18] Chen, C., Huang, T., Zhang, W., et al. (2020). ATP synthase complexome from photosynthetic bacterium Rhodobacter capsulatus. Nature Communications, 11(1), 2222.
[19] Storms, S. (2022). Outcomes of concomitant bedaquiline and delamanid in patients with multidrug-resistant tuberculosis. Infectious Disease Advisor. https://www.infectiousdiseaseadvisor.com
[20] Ushtanit, A., Mikhailova, Y., Lyubimova, A., et al. (2021). Genetic profile of linezolid-resistant M. tuberculosis clinical strains from Moscow. Antibiotics, 10(10), 1243. https://doi.org/10.3390/antibiotics10101243
[21] Lee, M., Lee, J., Carroll, M. W., et al. (2012). Linezolid for treatment of chronic extensively drug-resistant tuberculosis. New England Journal of Medicine, 367(16), 1508-1518. https://doi.org/10.1056/nejmoa1201964
[22] Diacon, A. H., Pym, A., Grobusch, M. P., de los Rios, J. M., Gotuzzo, E., Vasilyeva, I., Leimane, V., Andries, K., Bakare, N., De Marez, et al. & TMC207-C208 Study Group. (2014). Multidrug-resistant tuberculosis and culture conversion with bedaquiline. New England Journal of Medicine, 371(8), 723-732.
[23] Gler, M. T., Skripconoka, V., Sanchez-Garavito, E., Xiao, H., Cabrera-Rivero, J. L., Vargas-Vasquez, et al. (2012). Delamanid for multidrug-resistant pulmonary tuberculosis. New England Journal of Medicine, 366(23), 2151-2160.
[24] Huerga, H., Khan, U., Bastard, M., Mitnick, C. D., Lachenal, N., et al. and endTB study observational study team. (2022). Safety and Effectiveness Outcomes From a 14-Country Cohort of Patients With Multi-Drug Resistant Tuberculosis Treated Concomitantly With Bedaquiline, Delamanid, and Other Second-Line Drugs. Clinical Infectious Diseases, 75(8), 1307-1314. https://doi.org/10.1093/cid/ciac176
[25] Davidson, T., Lasky, J. A. M., & Frey, R. J. (2016). Tuberculosis. In D. S. Blanchfield (Ed.), The Gale Encyclopedia of Children's Health: Infancy through Adolescence (3rd ed., Vol. 4, pp. 2776-2781).
[26] Mondino, S., Gago, G., & Gramajo, H. (2013). Transcriptional regulation of fatty acid biosynthesis in mycobacteria. Molecular Microbiology, 89(2), 372-387. https://doi.org/10.1111/mmi.12282
[27] Hards, K., Robson, J. R., Berney, M., Shaw, L., Bald, D., Koul, A., Andries, K., & Cook, G. M. (2015). Bactericidal mode of action of bedaquiline. Journal of Antimicrobial Chemotherapy, 70(7), 2028-2037. https://doi.org/10.1093/jac/dkv054
[28] Harris, K. K., Fay, A., Yan, H. G., Kunwar, P., Socci, N. D., Pottabathini, N., et al. (2014). Novel imidazoline antimicrobial scaffold that inhibits DNA replication with activity against mycobacteria and drug-resistant Gram-positive cocci. ACS Chemical Biology, 9(11), 2572-2583. https://doi.org/10.1021/cb500573z
[29] Ehlers, S., & Schaible, U. E. (2013). The granuloma in tuberculosis: dynamics of a host-pathogen collusion. Frontiers in Immunology, 3, 411. https://doi.org/10.3389/fimmu.2012.00411
[30] Baykan, A. H., Sayiner, H. S., Aydin, E., Koc, M., Inan, I., & Erturk, S. M. (2022). Extrapulmonary tuberculosis: an old but resurgent problem. Insights into Imaging, 13(1), 39. https://doi.org/10.1186/s13244-022-01172-0
[31] Yuan, Y., & Barry, C. E., 3rd. (1996). A common mechanism for the biosynthesis of methoxy and cyclopropyl mycolic acids in Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America, 93(23), 12828-12833. https://doi.org/10.1073/pnas.93.23.12828
[32] Gler, M. T., Skripconoka, V., Sanchez-Garavito, E., Xiao, H., Cabrera-Rivero, J. L., Vargas-Vasquez, D. E., Gao, M., Awad, M., et al. (2012). Delamanid for Multidrug-Resistant Pulmonary Tuberculosis. New England Journal of Medicine, 366(23), 2151-2160.
[33] Hards, K., Robson, J. R., Berney, M., Shaw, L., Bald, D., Koul, A., Andries, K., & Cook, G. M. (2015). Bactericidal mode of action of bedaquiline. Journal of Antimicrobial Chemotherapy, 70(7), 2028-2037. https://doi.org/10.1093/jac/dkv054
[34] Alahari, A., Alibaud, L., Trivelli, X., Gupta, R., Lamichhane, G., Reynolds, R. C., Bishai, W. R., Guerardel, Y., & Kremer, L. (2009). Mycolic acid methyltransferase, MmaA4, is necessary for thiacetazone susceptibility in Mycobacterium tuberculosis. Molecular Microbiology, 71(5), 1263-1277. https://doi.org/10.1111/j.1365-2958.2009.06604.x
[35] Ushtanit, A., Mikhailova, Y., Lyubimova, A., Makarova, M., Safonova, S., Filippov, A., Borisov, S., & Zimenkov, D. (2021). Genetic Profile of Linezolid-Resistant M. tuberculosis Clinical Strains from Moscow. Antibiotics (Basel, Switzerland), 10(10), 1243. https://doi.org/10.3390/antibiotics10101243
[36] Gupta, R., Shuman, S., & Glickman, M. S. (2015). RecF and RecR Play Critical Roles in the Homologous Recombination and Single-Strand Annealing Pathways of Mycobacteria. Journal of Bacteriology, 197(19), 3121-3132. https://doi.org/10.1128/JB.00290-15
[37] Naidoo, K., Perumal, R., Ngema, S. L., Shunmugam, L., & Somboro, A. M. (2023). Rapid Diagnosis of Drug-Resistant Tuberculosis-Opportunities and Challenges. Pathogens (Basel, Switzerland), 13(1), 27. https://doi.org/10.3390/pathogens13010027
[38] Zou, Y., de Jager, V., Hesseling, A. C., Diacon, A. H., Wiesner, L., Mostert, J., Svensson, E. M., & Garcia-Prats, A. (2023). Relative bioavailability of delamanid 50 mg tablets dispersed in water in healthy adult volunteers. British Journal of Clinical Pharmacology, 10.1111/bcp.15672. Advance online publication. https://doi.org/10.1111/bcp.15672
[39] Zhang, Y., Zhu, H., Fu, L., Wang, B., Guo, S., Chen, X., Liu, Z., Huang, H., Yang, T., & Lu, Y. (2019). Identifying Regimens Containing TBI-166, a New Drug Candidate against Mycobacterium tuberculosis In Vitro and In Vivo. Antimicrobial Agents and Chemotherapy, 63(7), e02496-18. https://doi.org/10.1128/AAC.02496-18
[40] Yilmaz, F. F., Eraç, B., Ermertcan, Ş., Çavuşoğlu, C., Biçmen, C., Aktoğu Özkan, S., & Hoşgör Limoncu, M. (2018). In vitro effects of ciprofloxacin, levofloxacin and moxifloxacin on Mycobacterium tuberculosis isolates. Tuberculosis and Thorax, 66(1), 32-36.
[41] Dijkstra, J. A., van der Laan, T., Akkerman, O. W., Bolhuis, M. S., de Lange, W. C. M., Kosterink, J. G. W., van der Werf, T. S., Alffenaar, J. W. C., & van Soolingen, D. (2018). In Vitro Susceptibility of Mycobacterium tuberculosis to Amikacin, Kanamycin, and Capreomycin. Antimicrobial Agents and Chemotherapy, 62(3), e01724-17. https://doi.org/10.1128/AAC.01724-17
[42] Riccardi, N., Canetti, D., Rodari, P., Besozzi, G., Saderi, L., Dettori, M., Codecasa, L. R., & Sotgiu, G. (2020). Tuberculosis and pharmacological interactions: A narrative review. Current Research in Pharmacology and Drug Discovery, 2, 100007. https://doi.org/10.1016/j.crphar.2020.100007