by Dr. Boris Zakharov
ABOUT THE AUTHORS

Dr. Boris Zakharov
Professor Boris Zakharov began his professional career in the “Kedrovaya Pad Natural Reserve” (Far East State University, Vladivostok, Russia) as a research scientist, where he participated in and performed various scientific projects in field zoology. For five years, from 1974 to 1979, he collected invertebrates, processed zoological materials and monitored seasonal and long-term dynamics of natural ecosystems. For several years after that, Dr. Zakharov worked in the Institute of Agricultural Chemistry (Novosibirsk, Russia), where he received intensive and in-depth experience in the study of pest control and modern methods of crop protection. Dr. Zakharov's Doctoral research was on horse flies (Diptera, Tabanidae), beginning in 1983 at Novosibirsk Biological Institute. The results of this study were published in his papers and concern systematics, ecology, biogeography and cattle protection from blood sucking diptera in Siberia.
After his arrival in the United States, Dr. Zakharov participated in a project on ground spiders of Australia and New Zealand in the American Museum of Natural History, where he has worked from 1996 to 2005 as a Curatorial Assistant in the Spider laboratory in a Department of Invertebrate Zoology. Currently, he occupies the position of a visiting scientist at the American Museum of Natural History, where he continues the study of ground spiders of the world.
Professor Boris Zakharov began to teach in 2005 at Hostos Community College/CUNY. Boris joined the Natural Sciences department at LaGuardia in 2009.
The painful and potentially life-threatening condition known as decompression sickness, or the bends, occurs when gasses bubble out of the bodily fluids, forming air pockets in divers’ blood and organs when they ascend and external pressure on their body decreases. Divers may catch the bends in two different situations: 1) decompression sickness may occur when a diver uses a compressed air source, such as a scuba tank; or 2) during frequently repeated breath-hold dives. Both cases require separate analysis, but first, it is necessary to say something about gas exchange in mammals’ lungs.
The air is a mixture of many different gases including oxygen, nitrogen, carbon dioxide, and others. Together they create air pressure. All gases in the air contribute to air pressure according to the Dalton's law of partial pressure, which states that each gas in a mixture of gases exerts its own pressure, called partial pressure, proportional to its percentage in gaseous mixture and the total pressure of all gases is equal the sum of their partial pressures. The air usually contains 78% of nitrogen, 21% of oxygen, whereas carbon dioxide constitutes less than 1%. If the air pressure at sea level varies around 760 mm-Hg, then partial pressure of oxygen is PO2 = 760×0.21 = 160 mm-Hg, and nitrogen: PN2 = 760×0.78 = 593 mm-Hg. Gases move from the place where their concentration is high to areas, where their concentration is low. Because concentration of gases directly correlates with their partial pressure, one can state, that every gas moves from a place with high partial pressure to a place where its partial pressure is low. The higher gradient of partial pressure – the faster the gas moves. Alveoli are structures within the lungs that are covered by tiny film made of water and surfactant. Gases to diffuse across the respiratory membrane have to be dissolved in water. The ability of the gas to dissolve in water is called solubility. The degree at which gases dissolve in water is described by the Henry's law, which states that gas dissolves in water proportionally to its partial pressure and its solubility. This law explains the behavior of gases during the pulmonary gas exchange. For example, the partial pressure of nitrogen in the air is high, but its solubility in water is low. That is why normally there is much less nitrogen present in blood than oxygen, which has partial pressure that is much lower than that of nitrogen, but its solubility is significantly higher.
Compressed air from the scuba tank supplies lungs with regular air with 78% N2. The compressed air in the tank maintains the air pressure in the lungs that equalizes external pressure at depth. This prevents lungs of the diver to collapse under the force of hydraulic pressure and allows diver to breath. It means that the unusually high nitrogen partial pressure is continuously maintained in the lungs. For example, if the total pressure at depth is 3 atmospheres (atm), the PN2 is 2.3 atm; at total pressure 5 atm PN2 is almost 4 atm, and so on. High nitrogen partial pressure in lungs during diving leads to an increase of N2 in body tissues, until they reach equilibrium and blood, together with other body fluids, become saturated with this gas. During a fast ascend to the surface external pressure dramatically decreases and nitrogen gas in the bodily fluids creates macroscopic bubbles, the same way a gas forms bubbles in your bottle of soda, when you open it. These bubbles can block blood flow, press on nerve endings, and destruct proteins as a result of the change in pH (acidity) in gas-water interactions.
Breath-holding diving differs from diving with a scuba dive air tank by the fact that during descent, a diver’s lungs contain only a limited amount of air and a diver’s pulmonary supply with N2 is not renewed. The quantity of nitrogen in the lungs remains limited, and only this limited quantity can be transported to bodily fluids. The amount of N2 that is accumulated during a single breath-hold dive is too small to cause decompression sickness. However, if a diver does many repeated breath-hold descents and the periods between dives are not enough for his or her body tissues to have a chance to release and exhale accumulated N2 after each dive, the partial pressure of N2 steadily elevates and finally may reach a dangerous level sufficient to cause the bends.
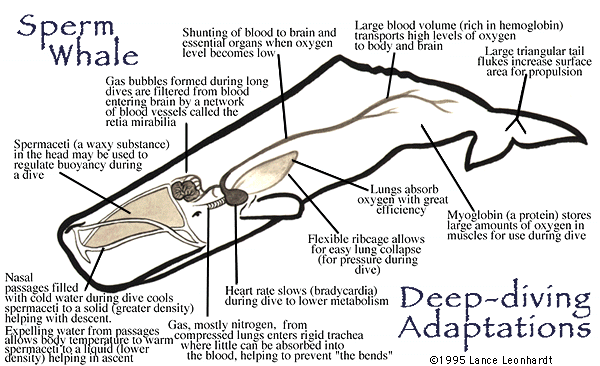
Whales and other marine mammals are breath-hold divers and it seems that they have some “secrets” to prevent nitrogen bubbles development and to avoid decompression sickness. There are three major mechanisms that protect these animals from decompression sickness: 1) special anatomic adaptation in their lungs and thoracic cage; 2) special changes in blood circulation during deep diving; and 3) behavioral adaptations that prevent frequent sequential diving.
Anatomy of marine mammals' chests allows their lungs to be compressed. Scientists have assumed that this passive compression was marine mammals' main adaptation to avoid taking up excessive nitrogen at depth and getting the bends. Their lung architecture forms two different pulmonary regions under deep-sea pressure. When air-breathing mammals dive to high-pressure depths, their lungs compress and alveoli collapse. Alveoli collapse keeps pulmonary N2 within the respiratory conduction system where there is no gas exchange between the air in the respiratory system and blood flow. This prevents buildup of N2 in the blood and bodily fluids. Compressibility of the thorax decreases the volume of the air steadily as the animal descends. If they ascend slowly, the nitrogen can return to the lungs and be exhaled. But if they ascend too fast, the nitrogen bubbles don't have time to diffuse back into the lungs. The depth at which the alveoli and alveolar sacs collapse completely is the depth at which the hydrostatic pressure is great enough to reduce the initial volume of lung air to the volume of the conducting airways. For example, if the volume of the conducting airways is 2 L, and if an animal carries 22 L of air in lungs on submergence, alveolar collapse should be complete at about 100 m, because at that depth the hydrostatic pressure is near 11 atm – sufficient to reduce the initial volume by factor of 11 to 2 L.
The circulation is another special adaptation of marine mammals to their life style. In 1870 Paul Bert found that diving animals decrease the rate of their heartbeat. This decrease of heart rate during diving was named diving bradycardia. Later it was also discovered that during diving the blood flow pattern of marine animals dramatically changes. Under the control of the sympathetic nervous system, blood vessels to most viscera, such as stomach, spleen, liver, pancreas, kidney, and so on constrict and these organs are cut off from blood supply. The parts of a diving mammal’s body that receive little or no blood flow include the animal’s limbs, the skeletal muscles of the trunk of its body, pectoral muscles, skin and body wall. At the same time, blood freely flows to the animal’s brain, lungs, and myocardium. Scientists describe it as the diving mammal becoming a “heart-lung-brain machine”. This alteration in heartrate and blood circulation prevents the accumulation of N2 in most animal body tissues. Researchers have taken CT images of a deceased dolphin, seal, and a domestic pig pressurized in a hyperbaric chamber. This study demonstrated that marine mammals' lung architecture creates two pulmonary regions: one air-filled and the other collapsed. The researchers believe that blood flows mainly through the collapsed region of the lungs. This causes what is called a ventilation-perfusion mismatch, which allows some oxygen to be absorbed by the animal's bloodstream, while minimizing or preventing completely the exchange of nitrogen.
Whale Songs is © 1996-2000 Lance Leonhardt and Black Box, © 2000-2004 Black Box.
Behavioral adaptations of marine animals include slow ascent during deep dives and prolonged lung ventilation after a series of fast shallow dives. As we can see, to prevent a buildup of N2 in body tissues and to avoid decompression sickness, a whale has to dive deep. However, in most cases marine animals prefer to dive repeatedly to depths that are too shallow to induce alveolar collapse. During shallow dives N2 might invade the blood and other tissues to a great extent enough to pose a risk of decompression sickness. Specialists in marine mammals think that animals simply avoid unsafe N2 partial pressures during normal diving by behaviorally limiting their diving schedule.
Recent studies of beaked whales have revealed new clues to the quest of deep diving marine mammals. Beaked whales are medium-sized toothed whales that inhabit depths beyond the continental shelf. Beaked whales have been catapulted into the spotlight by their tendency to “commit suicide” by beaching. Studies of deceased animals have shown that the presence of gas and fat emboli within the tissues and analysis of gas emboli is suggestive of nitrogen as the primary cause of such suicidal behavior. It was showed that adipose tissue plays a critical role in absorption of nitrogen gas from the blood flow. Anatomic regions containing lipid characterized by high solubility of nitrogen include acoustic fat bodies and accessory sinus system on the ventral regions of head. Robust arterial associations with lipid depots and air spaces in deceased animals were found within acoustic fat bodies of the lower jaw and pterygoid air sacs of the ventral head. These observations support the presupposition that these structures play an important role in the exchange of nitrogen gas during diving. They absorb nitrogen gas from blood flow and prevent bubble formation during ascent.
Scientists once thought that diving marine mammals were immune from decompression sickness. However, as it was mentioned above, it is not true and bends among marine mammals may occur in some circumstances. For example, now we know that beaked whales develop wide-spread bubble formation in their tissue after being exposed to high-intensity sonar signals of human origin. Thus, in 2002 stranding event linked to navy sonar exercises revealed that 14 whales died after beaching off the Canary Islands had gas bubbles in their tissues – a sign of the bends. A necropsy of those animals turned up evidence of damage from gas bubbles. The animals had stranded after exposure to sonar from nearby naval exercises. The sonar could destabilize dissolved in blood and body tissues nitrogen and force it to bubble.